Aaron Hudson, VP/GM, Clinical Diagnostics, SCIEX Aaron Hudson is Vice President and General Manager, Clinical Diagnostics at SCIEX. Aaron has been with SCIEX for over 17 years, beginning as a Sales Representative, and holding various roles including Director Global...
Tags

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.
High complexity of the lipidome
The complexity of the lipidome is diverse in the structure and there are many combinatorial isoforms that are available within nature. Currently, many different techniques are required to fully characterize a lipid molecule. What if you could do it in a single...

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.
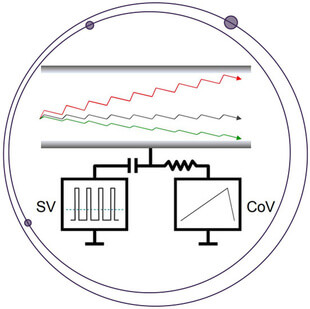
Elimination of Interference using the SelexION Differential Mobility System for the Quantitation of Rituximab in a Dual Surrogate Peptide Approach
The quantitation of proteins using the surrogate peptide approach can complicate nominal mass Triple Quadrupole MRM measurements due to co-extracted interference when using non-selective extraction techniques such as pellet digestion. High resolution coupled with accurate mass filtering can mitigate such interference, as reported previously for the determination of rituximab using the TripleTOF 6600 (Protein Quant Approaches). However, an additional level of selectivity can often be achieved on nominal mass systems using the orthogonal gas-phase separation approach offered by the SelexION+® Differential Mobility System technology (DMS). Interfaced between the sampling orifice and ion source, the DMS separates ions based upon differences in their migration rates under alternating low and high field waveform amplitudes (Figure 1). Ion clustering in low fields and declustering in high fields amplifies the distinction in mobility of an ion, resulting in improved resolution from interfering species of differing molecular cross-section.1-4

Enhancing In Vitro ADME Screening
LC-MS technology is helping contract research organization Cyprotex Discovery Ltd. perform bioanalysis of small molecules, peptides, and other pharmaceuticals, enabling quantification to be performed in complex matrices during in vitro ADME studies.
Host Cell Protein Analysis – Mass Spec’s Edge Over ELISA
The number of protein based drugs coming onto the market is at an all-time high, particularly those produced with a host cell system. With host cells come their own proteins. These host cell proteins (HCPs) constitute a major part of process-related impurities and can adversely affect drug safety, so it is critical that they are identified and quantified accurately.

Fipronil Tainted Eggs Detected in Several European Countries
News agencies all over the world are reporting a new food contamination issue regarding eggs which have been found to contain residues of Fipronil. According to Nieuwsuur, a Dutch news, and current affairs program, “The Fipronil scandal is a huge blow to the poultry sector. Millions of eggs are destroyed and 138 companies remain tentatively closed. But supermarkets also face great damage. In recent days all contaminated eggs have been taken out of the shelves.” CBS news has reported that contaminated eggs have been discovered in Belgium and in the Netherlands with other European countries now on alert.

In Search of the Unknown
The production of high-quality drinking water entails rigorous treatment and testing procedures. For water suppliers’ laboratories, such as the Zweckverband Landeswasserversorgung in Germany, one of the major challenges is the identification of trace levels of organic substances, which can be achieved with the help of mass spectrometry.

A Fleet of Analyzers Keeps Work Flowing
An Interview with Timothy Sangster, Head of Bioanalysis and Immunology, Charles River Laboratories, Edinburgh
Designed Specifically for Your Clinical Lab—Mass Spec Made Simple
Welcome to the first post in our clinical diagnostic blog series. Our ambition is to become your single destination for everything mass spec in the clinical diagnostic lab. To make this blog as useful as possible for you, we invite you to tell us what topics you would like us to cover. Please comment on this blog below and let us know what you’d like to hear!
Is Your Lab Prepared for Testing? The Global Supplement Market is Growing
Don’t judge a nutritional supplement by its label, as often, government monitoring of ingredients begins after the product enters the consumer market1. Meanwhile, there may be additional additives not mentioned on the label as they are used to address supplement side effects. Such is the case in the United States where even though federal law requires supplements to carry a dietary supplement label or a substitutional term, monitoring begins once a supplement is on the market. In China meanwhile, the China Food and Drug Administration’s (CFDA) health product potential illegal additives list, clearly stipulates monitoring processes for additives in six different types of nutritional supplements including weight loss, blood sugar reduction, blood pressure reduction, anti-fatigue, sleep improvement and immune strengthening functions.Read Tech Note >

How to Detect Additives in Cosmetics Amongst Ever Changing Regulations
In today’s technical blog, I’m talking about the cosmetics industry so let’s get right to it. According to a Research and Market report, “The Global Cosmetic market was $460 billion USD in 2014 and is estimated to reach 675 billion USD by 2020, growing at a rate of 6.4%.”1 The U.S. leads the pack with a reported $62 billion in revenue earned in 20162. So, what am I getting at? We know earnings are strong and consumers like their products. But the question remains, are these products that you put on your skin, hair, and ingest safe? Such is the thinking of scientists like me and other chemists who are routinely tasked with detecting minimal levels of potentially harmful ingredients in personal care products against ever-changing global regulations.

A Mine of Quantitative Proteomic Information
The Aebersold group at ETH Zurich focuses on proteomics research, including the development of techniques to study the proteome as an integrated entity. In collaboration with SCIEX, the group established SWATH® Acquisition mass spectrometry, a data-independent acquisition (DIA) method capable of fragmenting multiple peptide species concurrently. The resulting comprehensive data set can be retrospectively re-mined, enabling maximum benefit to be derived from any study.

Delivering New Biologics to the Marketplace
Characterization and quantification of host cell proteins (HCPs) in biopharmaceutical development and manufacturing is a critical step to ensuring product safety. While this can be achieved using ELISA, mass spectrometry using the SCIEX TripleTOF® 6600 System is more specific and enables the identification and quantitation of each of the individual proteins present.
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.

The Benefits of Using SWATH Acquisition Technology when Testing Pesticides in Food
Up until recently, SWATH® Independent Data Acquisition (IDA), was not widely used for the detection of pesticides in food samples. Introduced in 2012, SWATH Acquisition is an advanced acquisition technology capable of running on high-resolution mass spectrometers such as the X500R QTOF system or Triple TOF technology. Originally used in the Omics market to ID and quantify complex samples, SWATH Acquisition is gradually making a transition across markets including the investigation of pesticides in food. Like designer drugs, pesticides continuously undergo synthesizing, and food labs are beginning to require a more reliable analysis method to be confident in their resulting reports.

Single Injection, Routine Antibiotic Testing in Urine Samples
The consumption of pharmaceuticals and personal care products is a day to day occurrence. Once consumed the body excretes the remaining part of the compound which is not absorbed. This waste, flushed down the toilet, makes its way through the sewage system before arriving at a treatment facility where it was then processed with chemicals to ensure its cleanliness. Despite being washed, there can remain trace amounts of bacteria, hormones, metals, and antibiotics in whatever you consume, not just water
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.

Food Allergies – How Allergic Are You?
A recent study published by the Annals of Allergy, Asthma, and Immunology (ACAAI), pointed out, in a study of 109 people tested, that skin prick tests are not 100 percent reliable. In the study, participants were subjected to oral food challenges prior to skin testing in which 50 percent of individuals had no reaction. It was also discovered that blood tests were not full-proof even though they measure the presence of IgE antibodies to specific foods. These results are not surprising given that 50 to 60 percent of tests result in false-positives.
Comprehensive Therapeutic Protein Characterization Using One Single Method
Get to know how CESI-MS will allow you to quickly and accurately characterize protein therapeutics for attributes in a single method by downloading this discovery kit.

A Fresh Approach to Food Safety
The EU Reference Laboratory (EURL) for Fruits and Vegetables in Almería is responsible for a network of around 200 laboratories which provide essential surveillance and monitoring to ensure the safety of foodstuffs available across Europe. The EURL provides proficiency testing and method development for these official laboratories, ensuring rigorous screening to avoid harmful chemicals entering the food chain
The Only Solution You Need for Fast MetID
If you work in the breakneck world of therapeutic development, then you probably don’t even have time to read this blog (but we thought we would write it anyways, just in case). Drug metabolism samples are coming into your lab fast and furious. You need to turn them around in hours so that chemists and biologists can optimize the effectiveness of the therapeutic candidate. Time for lunch? We don’t think so!

The Key to Measuring Chemical Dyes in Food is LC-MS/MS
Adding colorful dyes to food is nothing new. In the early 19th century, for example, it wasn’t uncommon for manufacturers to add chalk to white bread, thicken milk with a lead compound, and inject red dye into meat in the quest for a fresher appearance1. Fast forward to the 21st century, however, and along with mass spectrometry, food standards have come a long way. Foods now must pass muster according to standards set by government regulators or else risk fines and punishment which can be costly for the manufacturer. To support these measures, are agencies such as the US-FDA, EFSA, and others which have banned some colors due to their toxic and carcinogenic nature which brings me to mass spectrometry analysis. Discover more when you read the following application note, “LC-MS/MS Analysis of Emerging Food Contaminants,” in which researchers used the ExionLC AD with a Phenomenex Column for sample separation followed by MS/MS detection with the SCIEX X500R QTOF system.