 Read time: 4 minutes
Read time: 4 minutes
In this final installment of our “Back to the new basics” series, we take one more look at analytical techniques and best practices in the lab, and opportunities to improve efficiency. Here, we explore the basic principles of high-performance liquid chromatography (LC) and liquid chromatography mass spectrometry (LC-MS), and how these techniques can affect a lab’s efficiency and productivity.
What is LC?
LC is a separation technique that relies on a partitioning of compounds between a liquid mobile phase and a solid stationary phase contained inside an LC column. When samples are injected, they are absorbed on the stationary phase. The solvent then passes through the column and compounds desorb from the stationary phase based on their relative affinity to the packing materials in the varying solvent composition. The compound with the most affinity to the stationary phase is the last to be released as it requires the highest concentration of the eluting solvent to be removed from the column. Reverse phase chromatography is commonly used in combination with MS to achieve this separation. With this technique, compounds stick to the stationary phase based on their hydrophobicity and are eluted using a gradient of increasing organic solvent.
LC is used in various applications for both qualitative and quantitative applications in pharmaceuticals and in environmental and food analyses. Many labs use it for quantifying low or non-volatile organic compounds, such as vitamins in food and pesticides in water or food products.
What about LC-MS?
With LC, an optical detection system is typically used. LC can also easily be coupled with an MS system for detection (Figure 1). An LC-MS detection system has advantages over optical detection as it has increased specificity for the target compounds and often has improved sensitivity.
Since the development of electrospray ionization (ESI) in 1984, coupling LC to MS has become a routine technique in analytical labs. ESI provides a simple and robust interface that makes LC-MS useful in applications that analyze large numbers of compounds and biological molecules. Another reason for its adoption is its ability to achieve high sensitivity and accuracy when used with tandem MS (LC-MS/MS), which adds specificity for compound detection in complex matrices. In addition, the faster scanning speeds of newer LC-MS systems allows a higher degree of multiplexing, which enables many more compounds to be measured within a single analytical run.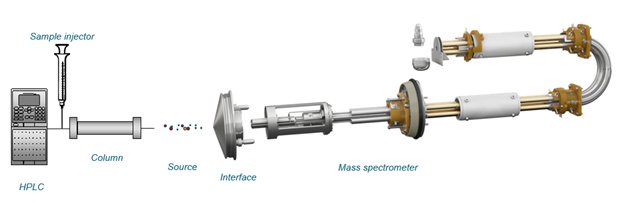
Figure 1. Overview of an LC-MS instrument.
LC vs. LC-MS: an analysis
Busy labs need to boost efficiency and increase their throughput but still maintain or improve their performance standards. While LC alone will provide precise results, it can have efficiency costs—for example, the sample prep is cumbersome and complicated, and gradients can often be long to get to the desired level of specificity. To estimate the true cost of an LC-based method and its effect on your lab’s productivity, you must factor in the chemicals needed, the time required and the necessary human resources. In addition, in food testing, traditional assays can produce false negatives and positives due to limited sensitivity and selectivity, forcing retests.
LC-MS delivers more power and opens your lab up to a greater variety of testing. The strength of LC-MS lies in the separation power of LC for a wide range of compounds combined with the ability of MS to quantify compounds with a high degree of sensitivity and selectivity. LC-MS can even handle many compounds that are difficult for gas chromatography mass spectrometry (GC-MS) to analyze, such as thermally labile and chemically unstable analytes, amines and semi-volatile compounds. It can also save time by condensing multiple test methods into a single analysis, enabling you to classify more analytes in a single run.
Consider this study, where LC-MS was used to develop a quantitative analysis of antiepileptics (carbamazepine and phenytoin) and beta-blocking drugs (acebutolol, atenolol, pindolol and propranolol). The results showed that LC-MS was faster, more sensitive and more specific when compared to LC. LC-MS was also able to analyze incompletely resolved mixtures and achieve even lower absolute detection limits than HPLC.
Getting started
All this techy talk can get overwhelming, especially if you are just looking for ways to get the job done without burning a hole in your pocket. So how can you easily and cost effectively get started with LC-MS in your own lab?
The SCIEX Triple Quad 3500 system is an entry-level MS designed for modern analytical laboratories. It provides ultra-fast detection and high sensitivity to enable comprehensive multi-component analysis. Don’t just take my word for it, download the SCIEX Triple Quad 3500 system compendium to learn more about what it can do for your laboratory.
If you require additional training, check out the online and in-person courses available in the SCIEX Now Learning Hub to help get your lab up to speed. And be sure to take advantage of our new digital tool, the SCIEX Now Learning Manager, which provides a centralized digital platform that enables lab directors to spend less time and resources tracking the training competencies of their staff and offers access to current content from leading industry experts at SCIEX. Browse and enroll now!
This is part 3 of a 3-part “Back to the new basics” series on mass spectrometry.
Part 1: Making the leap from GC-MS to LC-MS
Part 2: Do I need a single quadrupole or a triple quadrupole system?
RUO-MKT-18-14201-A






 Contact Support
Contact Support
0 Comments