We know that LC-MS oligonucleotide analysis can have its share of challenges—challenges with sensitivity, challenges with adduct formation and challenges with data analysis, to name just a few. That’s why this blog takes a closer look at the dos and don’ts of this type of analysis and explores some keys to success. It also explains why following these simple rules can vastly improve your oligonucleotide characterization and quantitation efficiency and success.
Tags

The whys behind the dos and don’ts of oligonucleotide analysis
We know that LC-MS oligonucleotide analysis can have its share of challenges—challenges with sensitivity, challenges with adduct formation and challenges with data analysis, to name just a few. That’s why this blog takes a closer look at the dos and don’ts of this type of analysis and explores some keys to success. It also explains why following these simple rules can vastly improve your oligonucleotide characterization and quantitation efficiency and success.
Top 7 Echo® MS system customer questions—answered
You asked, we answered! With analysis speeds of at least 1 sample per second, the Echo® MS system has created a buzz in the industry. This is up to 50x faster than conventional LC-MS/MS. This revolutionary tool for drug discovery and development has led to...

An interview from the Science Explorer about the Echo® MS system
The Science Explorer interviews Neil Walsh from SCIEX to discuss the significant application areas of the Echo® MS System and what makes this system so attractive to biopharmaceutical laboratories.

The Echo® MS system: Is it reproducible? Yes… yes… yes!
The Echo® MS System is an exciting new platform that dramatically speeds sample analysis for quantitative MS studies. Because of its unique and innovative technology, the system can analyze samples faster than ever before—but without the need for LC.

Scale it up! The Echo® MS System delivers unprecedented levels of productivity
Imagine the productivity gains your lab could achieve with a technology that not only analyzes samples up to 50x faster than conventional quantitative LC-MS, but also eliminates tedious sample preparation, time-consuming LC method development and chromatographic run...

How fast is fast? The Echo® MS System sets the record
How fast is fast? Cheetahs. Usain Bolt. Tachyons. The Echo® MS system. What do these things have in common? They’re all fast. REALLY fast. In fact, they’re the fastest in their categories: the fastest land mammal, the fastest human sprinter, the fastest subatomic...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.
Keep Those Interactions Intact and Analyze the True Nature of Your Protein Compounds
There’s no doubt that mass spectrometry has emerged as an essential technique for drug discovery and development. But the denaturing conditions of conventional LC-MS will typically break apart any non-covalent interactions that exist in solution – even though there is...

Ever thought of breaking the high-throughput sound barrier for drug discovery?
Wouldn’t it be great if we really could “get time back” or even “buy time”? When developing pharmaceuticals, it takes years to bring a new therapy to the market due to the linear nature of the process. As the saying goes, “Time waits for no one.” But what if we could...
Why Conventional Flow LC-MS/MS Bioanalysis Is out and Microflow Is In
New technologies can transform a laboratory’s throughput and efficiency. At Alturas, if we try out a new technology, we ask: Does it work? Is it convenient? Is it rugged? Are we getting good results? When we look at the convenience, uptime, and overall data that we’re...

What is Multi Attribute Methodology (MAM)?
Q&A with Sean McCarthy Global Market Manager, Biologics, SCIEX MAM is an acronym for Multiple Attribute Method. In short, MAM is a method which may be applied for characterization of a biotherapeutic to understand its sequence, identify liabilities, identify...

New Methods for N-Glycan Sequencing Provide Structural Information in as Little as 1 Hour
As interest in N-glycan analysis grows within the biopharma industry, innovation continues allowing analyses to be done in less time with fewer and less tedious, sample preparation steps. One of these recent innovations was the release of the SCIEX Fast Glycan...

Push Your Research to the Cutting Edge: 2018 Global CESI-MS Symposium
There are a lot of conferences vying for your attention every year. The Global CESI-MS Symposium on October 10-11 in the Netherlands is one you can’t afford to miss. It is the place to hear about the latest advancements made through the adoption of CESI-MS...

Save Effort. Save Time. Consolidate Your BioTherapeutic Quality, Safety, and Efficacy Assessments with One Direct LC-MS MAM Assay.
In a previous blog, we learned how you could Simplify Your Life with a Streamlined Workflow for Multiple Attribute Methodology (MAM). Based on the well-known technique of peptide mapping, a Liquid Chromatography-Mass Spectrometry-based MAM workflow (LC-MS MAM) enables...

Biologics Characterization: Intact and Subunit Mass Analysis
When All You Need Is Range – Mass Range, Dynamic Range, and the Complete Range Launching the best possible product in the shortest time possible is key for pharmaceutical companies like you. As you know, nearly every process throughout the biologics development cycle...

Product Quality Assessments of Biotherapeutics Using LC-MS MAM
Why LC-MS MAM?The Liquid Chromatography-Mass Spectrometry Multiple Attribute Methodology (LC-MS MAM) is a technique that is quickly gaining traction in the development and manufacturing of biopharmaceuticals. But what exactly is MAM? As researchers describe in a...

Simplify Your Life with a Streamlined Workflow for Multiple Attribute Methodology (MAM)
The effort to fully characterize and release a biotherapeutic to the market can be onerous. Typically, many tests are required to identify and monitor various attributes of the final product in order to ensure the safety and efficacy of the drug. These product quality...

Calling all Bioanalysts: We’re Making Bioanalytical Selectivity Challenges History
High selectivity is a key component of successful quantitative bioanalysis. As a bioanalyst, we need consistently accurate and robust quantitation of small molecule therapeutics and metabolites. Challenged by complicated matrix interferences, high baseline signal, and...
The Ultimate Selectivity for Peptide and Protein Quantitation
There’s no doubt about it, biopharma drug development is experiencing phenomenal growth and presents a variety of challenges not experienced in small molecule development. Some of these challenges are in the selective and sensitive quantitation of peptides and...

On Demand Videos from the 2017 Global CESI-MS Symposium
The 2017 Global CESI-MS Symposium brought together KOLs and industry innovators from around the world to share their latest advancements using capillary electrophoresis integrated with electrospray ionization (CESI-MS) within the same device.
3 Workflows Designed to Accelerate Your Biologics Characterization
Biopharmaceutical development is booming and now an integral part of many pharmaceutical company pipelines. While these emerging biologics present exciting opportunities for the industry, their sophistication is challenging the limits of characterization at all stages of discovery and development.
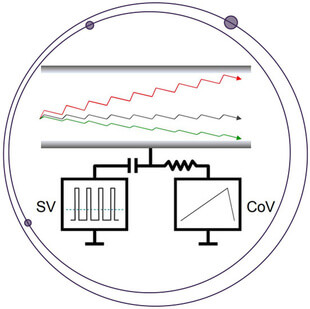
Elimination of Interference using the SelexION Differential Mobility System for the Quantitation of Rituximab in a Dual Surrogate Peptide Approach
The quantitation of proteins using the surrogate peptide approach can complicate nominal mass Triple Quadrupole MRM measurements due to co-extracted interference when using non-selective extraction techniques such as pellet digestion. High resolution coupled with accurate mass filtering can mitigate such interference, as reported previously for the determination of rituximab using the TripleTOF 6600 (Protein Quant Approaches). However, an additional level of selectivity can often be achieved on nominal mass systems using the orthogonal gas-phase separation approach offered by the SelexION+® Differential Mobility System technology (DMS). Interfaced between the sampling orifice and ion source, the DMS separates ions based upon differences in their migration rates under alternating low and high field waveform amplitudes (Figure 1). Ion clustering in low fields and declustering in high fields amplifies the distinction in mobility of an ion, resulting in improved resolution from interfering species of differing molecular cross-section.1-4
Host Cell Protein Analysis – Mass Spec’s Edge Over ELISA
The number of protein based drugs coming onto the market is at an all-time high, particularly those produced with a host cell system. With host cells come their own proteins. These host cell proteins (HCPs) constitute a major part of process-related impurities and can adversely affect drug safety, so it is critical that they are identified and quantified accurately.

A Fleet of Analyzers Keeps Work Flowing
An Interview with Timothy Sangster, Head of Bioanalysis and Immunology, Charles River Laboratories, Edinburgh

Delivering New Biologics to the Marketplace
Characterization and quantification of host cell proteins (HCPs) in biopharmaceutical development and manufacturing is a critical step to ensuring product safety. While this can be achieved using ELISA, mass spectrometry using the SCIEX TripleTOF® 6600 System is more specific and enables the identification and quantitation of each of the individual proteins present.
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.
Comprehensive Therapeutic Protein Characterization Using One Single Method
Get to know how CESI-MS will allow you to quickly and accurately characterize protein therapeutics for attributes in a single method by downloading this discovery kit.



 Contact Support
Contact Support