As interest in N-glycan analysis grows within the biopharma industry, innovation continues allowing analyses to be done in less time with fewer and less tedious, sample preparation steps. One of these recent innovations was the release of the SCIEX Fast Glycan...
Tags

The whys behind the dos and don’ts of oligonucleotide analysis
We know that LC-MS oligonucleotide analysis can have its share of challenges—challenges with sensitivity, challenges with adduct formation and challenges with data analysis, to name just a few. That’s why this blog takes a closer look at the dos and don’ts of this type of analysis and explores some keys to success. It also explains why following these simple rules can vastly improve your oligonucleotide characterization and quantitation efficiency and success.
Top 7 Echo® MS system customer questions—answered
You asked, we answered! With analysis speeds of at least 1 sample per second, the Echo® MS system has created a buzz in the industry. This is up to 50x faster than conventional LC-MS/MS. This revolutionary tool for drug discovery and development has led to...

An interview from the Science Explorer about the Echo® MS system
The Science Explorer interviews Neil Walsh from SCIEX to discuss the significant application areas of the Echo® MS System and what makes this system so attractive to biopharmaceutical laboratories.

The Echo® MS system: Is it reproducible? Yes… yes… yes!
The Echo® MS System is an exciting new platform that dramatically speeds sample analysis for quantitative MS studies. Because of its unique and innovative technology, the system can analyze samples faster than ever before—but without the need for LC.

Scale it up! The Echo® MS System delivers unprecedented levels of productivity
Imagine the productivity gains your lab could achieve with a technology that not only analyzes samples up to 50x faster than conventional quantitative LC-MS, but also eliminates tedious sample preparation, time-consuming LC method development and chromatographic run...

How fast is fast? The Echo® MS System sets the record
How fast is fast? Cheetahs. Usain Bolt. Tachyons. The Echo® MS system. What do these things have in common? They’re all fast. REALLY fast. In fact, they’re the fastest in their categories: the fastest land mammal, the fastest human sprinter, the fastest subatomic...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.

Ever thought of breaking the high-throughput sound barrier for drug discovery?
Wouldn’t it be great if we really could “get time back” or even “buy time”? When developing pharmaceuticals, it takes years to bring a new therapy to the market due to the linear nature of the process. As the saying goes, “Time waits for no one.” But what if we could...

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.
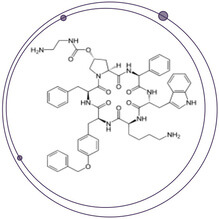
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...
Harnessing the Power of MRM3 for Large Molecule Quantitative Bioanalysis
In a previous blog outlining the advantages of high-resolution accurate mass measurements for protein quantitation using the TripleTOF 6600, it was noted that although the triple-stage quadrupole demonstrated high sensitivity when operated in multiple reaction monitoring mode (MRM), the relatively low-resolution measurement of m/z failed to discriminate Rituximab response from nominally isobaric interferences given the complexity of the proteolytically digested samples (June 28/2016). While the accurate mass filtering capabilities of the TripleTOF 6600 represents one mechanism for achieving increased selectivity over MRM, the triple quadrupole/linear ion trap (LIT) hybrid platform represented by the QTRAP® 4500, 5500, 6500 and 6500+ systems provides an alternative technique by leveraging a third stage of MS, often referred to as MRM3. In this blog, we outline the MRM3 scan function and survey several large molecule applications which utilize the additional stage of fragmentation in the LIT to yield significant improvements in achievable detection limits when compared to MRM.
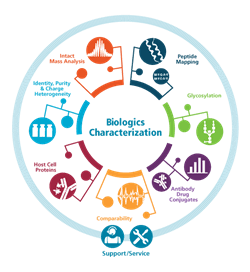
See What More You Can do With 360 Degree Biologics Characterization
Ever wish you had your own team of mass spectrometry experts at your side when working through biologics development and characterization challenges? With SCIEX 360° Innovations complex biologics characterization is streamlined with a full suite of mass spectrometry (MS), capillary electrophoresis (CE) systems, software, and services from SCIEX experts.

You’ve Seen It… Now Try It! BioPharmaView Software 2.0
At ASMS this year, the newest version of BioPharmaView Software was released. This software simplifies the processing of biotherapeutic data for characterization and comparability which can dramatically improve your productivity. BioPharmaView 2.0 Software accelerates characterization and comparability studies and simplifies reporting, so you can make better decisions, faster.

Three Infographics to Show You How to Overcome Challenges in Transitioning to Biologics Bioanalysis
The move to large molecules in Pharma is accelerating, offering unprecedented opportunities to improve human health and expand into new markets. But for those with extensive experience with small molecule bioanalysis, the shift to biologics can be challenging, from Sample Prep to Instrumentation and Software, to Methods and Training:

Rapid Characterization of Biologics using CESI-MS
Today, 30 monoclonal antibodies (mAbs), have been approved for the treatment of certain cancers, autoimmune and infectious diseases. Even more are in development, and perhaps you and your team of scientists are working on one now. Keeping pace with fast development timelines while performing comprehensive characterization of biologic candidates can be challenging. However, more and more, scientists are tackling these challenges with new techniques to speed and simplify their characterization workflows. Read more in the application note, “Rapid Characterization of Biologics using a CESI 8000 – SCIEX TripleTOF® System,” found in the Biologics Analytical Characterization Compendium, which highlights how CESI separation coupled with high-resolution mass spectrometry can provide a comprehensive characterization of biotherapeutics.
Protein Quantitation Workflows using the TripleTOF 6600: A Case Study for Rituximab
Although the triple-stage quadrupole (QQQ) mass spectrometer remains the pillar for quantitative LC-MS/MS bioanalytical assays, due in part to the platforms’ high duty cycle when operated in multiple-reaction monitoring (MRM) mode, the applicability of high-resolution mass spectrometry (HRMS) has become of increasing importance for protein quantitation given the complexity of proteolytically digested samples in the surrogate peptide approach. While the QQQ demonstrates high sensitivity and specificity, the relatively low-resolution measurement of m/z may fail to differentiate analyte response from nominally isobaric background interference. In contrast, HRMS with accurate mass assignment of product ion allows interference to be resolved through judicious selection of a post-acquisition mass extraction window whose tolerance is largely dictated by the effective resolution and stability of mass calibration.

Fast, Efficient, Disulfide Bond Mapping Using BioPharmaView™ Software
Fast LC-MS acquisition and automated data processing will help you speed up peptide mapping of your biotherapeutic, including critical disulfide bond and post-translational modification characterization. SCIEX helps you untangle the complexity of disulfide bonds, speeding up your characterization process.

Characterize and Monitor Host Cell Proteins (HCPs) Using SWATH Acquisition Technology
During drug development, the removal of impurities and purification of a final drug product is absolutely essential in order to ensure the safety and efficacy of a therapeutic drug. Of particular concern for biologics are impurities that can stem from host cell proteins. Because biologics are developed through cell culture and fermentation within a host cell, proteins from this host cell can be co-purified with the final biologic. These host cell proteins or HCPs can cause the final product to have undesired side-effects such as eliciting an immune response in patients taking the drug, or affecting the drug’s stability or efficacy. As a result, regulating agencies require drug companies to monitor levels of HCPs during the development and purification of a biologic and to remove HCPs to an acceptable level in the final biotherapeutic product.
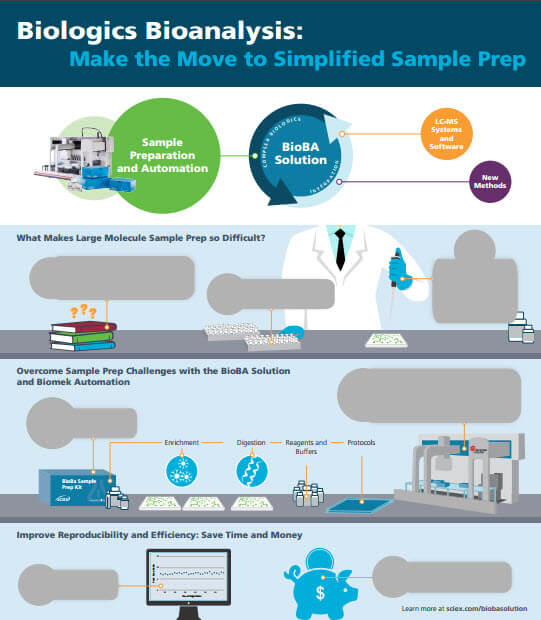
Simplifying Biologics Bioanalysis Sample Prep
These days, everyone seems to be furiously scratching tickets to become instant winners, but I’ll bet you didn’t expect to find sample prep tips that way. For large molecule bioanalysis, preparing your samples can be one of the biggest challenges. It’s a whole different world from traditional small molecule bioanalysis. SCIEX has developed techniques and automation that make biologics sample prep simpler and faster, with reproducible results.
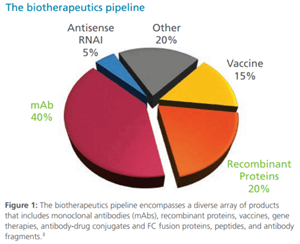
The Future of Biologics Drug Development is Today
Since the 1982 approval of Eli Lilly’s recombinant human insulin, Humulin, biotherapeutic drug development has steadily grown into a global market valued at $140 billion in 2013, increased from $25 billion in 2001
A Hybrid LBA/LC-MS Assay – Your Questions Answered
Last week we posted a blog on Biologics Bioanalysis Key Challenges, where we presented a webinar on those key challenges.

LC-MS/MS Method for Biotherapeutic Drug Development Challenges
Traditionally, the pharmacokinetic profile of biotherapeutics such as insulin glargine, adalimumab, trastuzumab and others, used gold standard LBAs to assess dose-response during drug discovery and development. However, LBAs require a specific antibody reagent to be developed for each mAb variant, a process that is often incompatible with the compressed timeframes encountered during the initial stages of drug development.