The “Intensity (Peptide)” values come from LCMSReconstruct, in ProteinPilot software 5.0. It maps the RT, m/z, intensity MS1 surface to find the peak information for the peptide. The Intensity (Peptide) is a weighted sum of the heights of the isotope series at the...
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.

Metabolite identification and peace of mind
Managing metabolite identification (Met ID) studies is challenging, so what is at the top of your priority list as you plan the year ahead? Ensuring you have the data needed to manage product safety, meeting deadlines, staff recruitment and training, maintaining compliance, capital expenses, or something else?

What has the Echo® MS system done for the pharma industry? (And don’t just take our word for it!)
SCIEX was very proud to have an illustration of the Acoustic Ejection Mass Spectrometry (AEMS) technology that powers the Echo® MS system on the front cover of the Journal of the American Society for Mass Spectrometry in January 2023. The associated article—Ultrahigh-Throughput Intact Protein Analysis with Acoustic Ejection Mass Spectrometry—was co-authored by scientists from SCIEX and Merck.

New features in OneOmics suite
I just wanted to thank the readers here, both the OneOmics suite users who’ve shared their time and watched OneOmics grow, and for all the talented developers and scientists who’ve made OneOmics suite what it is today.
The History of Isotopic Labels for Quantitative Proteomics
Proteomics has become a vital tool for biological scientists performing research on the healthy and diseased states of living things. It involves the large scale and systematic analysis of all proteins within a given cell, tissue, or organism. Because proteins are regulated by many different internal and external stimuli, the proteome is dynamic and quantities of proteins can change from one state to the next. Therefore, in order to be of the highest utility, proteomics experiments need to both identify and quantify proteins so that comparative studies can be done, such as between healthy cells and tumor cells, or the comparison of different treatment regimens.

Taking care of your mass spectrometer—Onsite troubleshooting and maintenance training for today’s lab
Recently, we asked customers to tell us about their biggest challenges so we could customize training programs to meet the needs of today’s growing lab. Without hesitation, most of you said uptime and employee training are your most critical needs. As a result, our...
The Promise of Precision Medicine
Here is the latest update on the Worldwide Efforts to Accelerate Precision Medicine
The NIH recently issued a press release in early July announcing $55 million in awards. According to the release, the $55 million award in the fiscal year 2016 will go towards building the foundational partnerships and infrastructure needed to launch the Cohort Program of President Obama’s Precision Medicine Initiative (PMI). The PMI Cohort Program is a landmark longitudinal research effort that aims to engage 1 million or more U.S. participants to improve the ability to prevent and treat disease based on individual differences in lifestyle, environment, and genetics.
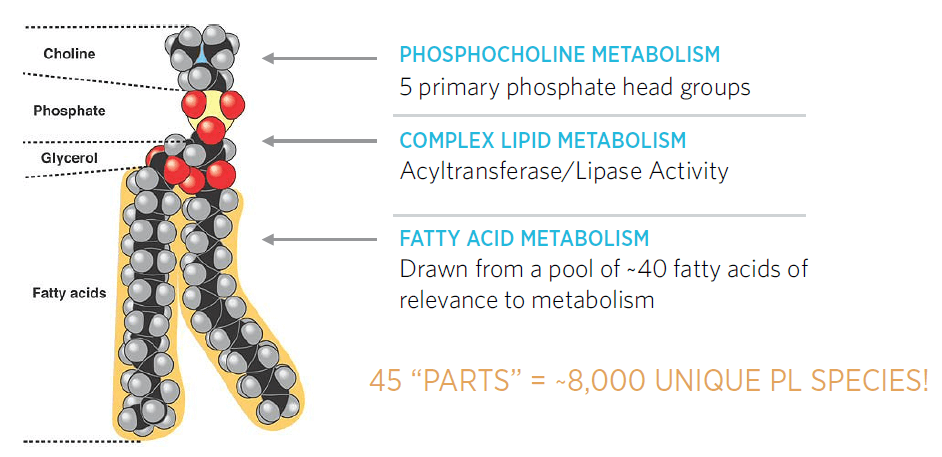
Why Study Lipids?
I had an opportunity to follow up with Steven M Watkins, Ph.D. to talk about the importance of studying lipids in disease. Steve has been working in the lipids field for over 20 years and is one of the foremost experts in lipid biology. Steve founded Lipomics in 2000, an early metabolomics company focused on quantitative lipidomics and had followed that company through a series of changes that led to its involvement in the clinical diagnostic development and global metabolomics. Steve authored over 70 peer-reviewed publications including several book chapters on lipids and lipid metabolism. His presentations on this topic are fascinating and very informative, so I wanted to capture some of his thinking here!

Improved complex sample processing for higher quality of results, reproducibility and depth of proteomic analysis
SCIEX partners to improve depth of proteome coverage
SCIEX and Pressure BioSciences address a major challenge for researchers performing complex sample preparation by marketing a complete solution to increase the depth, breadth, and reproducibility of protein extraction, digestion, and quantitation in all tissue types, especially challenging samples like tumors.
Industrialize Your Quantitative Proteomics Using a More Simplified Sample Prep
in part 1 and part 2 of this blog series we discussed how you can increase your efficiency for high throughput quantitative proteomics by industrializing your sample analysis and data processing. Microflow SWATH® Acquisition on your TripleTOF® system coupled with OneOmics™ data analysis tools allow you to run samples faster, collect data faster, and process your data files faster. It all adds up to getting more meaningful biological information in a shorter amount of time.

Industrialize Your Quantitative Proteomics with the OneOmics Project
For many labs, the days are long gone when it was acceptable to run only a few samples a week for your quantitative proteomics projects. The pressure for faster turn-around times, to support larger cohort studies, to sustain multiple research directions, and to transition from a purely unbiased discovery mode to verifying something truly unique and interesting, all demand a faster pace. Many labs are now being asked to analyze a hundred samples a week or more. In part 1 of this blog series, we saw how moving to a microflow SWATH workflow can dramatically increase your throughput with little compromise on overall results. In this part, we’ll address what to do with all of this data because it’s just no good if all we’ve done is move the bottleneck downstream.
Taking on Precision Medicine with Industrialized Proteomics
What if we could deliver the right treatment at the right time, to the right person to better, more effectively treat complex disease? This is the promise of precision medicine, to be able to approach complex disease treatment and prevention by taking into account individual variability in genes, environment, and lifestyle for each person.
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.