Imagine the productivity gains your lab could achieve with a technology that not only analyzes samples up to 50x faster than conventional quantitative LC-MS, but also eliminates tedious sample preparation, time-consuming LC method development and chromatographic run...
Tags

The whys behind the dos and don’ts of oligonucleotide analysis
We know that LC-MS oligonucleotide analysis can have its share of challenges—challenges with sensitivity, challenges with adduct formation and challenges with data analysis, to name just a few. That’s why this blog takes a closer look at the dos and don’ts of this type of analysis and explores some keys to success. It also explains why following these simple rules can vastly improve your oligonucleotide characterization and quantitation efficiency and success.
Top 7 Echo® MS system customer questions—answered
You asked, we answered! With analysis speeds of at least 1 sample per second, the Echo® MS system has created a buzz in the industry. This is up to 50x faster than conventional LC-MS/MS. This revolutionary tool for drug discovery and development has led to...

An interview from the Science Explorer about the Echo® MS system
The Science Explorer interviews Neil Walsh from SCIEX to discuss the significant application areas of the Echo® MS System and what makes this system so attractive to biopharmaceutical laboratories.

The Echo® MS system: Is it reproducible? Yes… yes… yes!
The Echo® MS System is an exciting new platform that dramatically speeds sample analysis for quantitative MS studies. Because of its unique and innovative technology, the system can analyze samples faster than ever before—but without the need for LC.

Scale it up! The Echo® MS System delivers unprecedented levels of productivity
Imagine the productivity gains your lab could achieve with a technology that not only analyzes samples up to 50x faster than conventional quantitative LC-MS, but also eliminates tedious sample preparation, time-consuming LC method development and chromatographic run...

How fast is fast? The Echo® MS System sets the record
How fast is fast? Cheetahs. Usain Bolt. Tachyons. The Echo® MS system. What do these things have in common? They’re all fast. REALLY fast. In fact, they’re the fastest in their categories: the fastest land mammal, the fastest human sprinter, the fastest subatomic...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.

Ever thought of breaking the high-throughput sound barrier for drug discovery?
Wouldn’t it be great if we really could “get time back” or even “buy time”? When developing pharmaceuticals, it takes years to bring a new therapy to the market due to the linear nature of the process. As the saying goes, “Time waits for no one.” But what if we could...

On Demand Videos from the 2017 Global CESI-MS Symposium
The 2017 Global CESI-MS Symposium brought together KOLs and industry innovators from around the world to share their latest advancements using capillary electrophoresis integrated with electrospray ionization (CESI-MS) within the same device.
3 Workflows Designed to Accelerate Your Biologics Characterization
Biopharmaceutical development is booming and now an integral part of many pharmaceutical company pipelines. While these emerging biologics present exciting opportunities for the industry, their sophistication is challenging the limits of characterization at all stages of discovery and development.
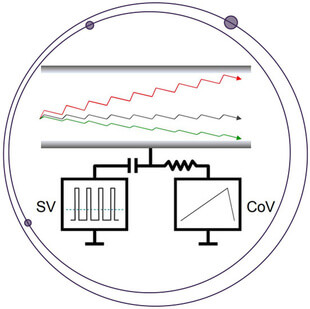
Elimination of Interference using the SelexION Differential Mobility System for the Quantitation of Rituximab in a Dual Surrogate Peptide Approach
The quantitation of proteins using the surrogate peptide approach can complicate nominal mass Triple Quadrupole MRM measurements due to co-extracted interference when using non-selective extraction techniques such as pellet digestion. High resolution coupled with accurate mass filtering can mitigate such interference, as reported previously for the determination of rituximab using the TripleTOF 6600 (Protein Quant Approaches). However, an additional level of selectivity can often be achieved on nominal mass systems using the orthogonal gas-phase separation approach offered by the SelexION+® Differential Mobility System technology (DMS). Interfaced between the sampling orifice and ion source, the DMS separates ions based upon differences in their migration rates under alternating low and high field waveform amplitudes (Figure 1). Ion clustering in low fields and declustering in high fields amplifies the distinction in mobility of an ion, resulting in improved resolution from interfering species of differing molecular cross-section.1-4
Host Cell Protein Analysis – Mass Spec’s Edge Over ELISA
The number of protein based drugs coming onto the market is at an all-time high, particularly those produced with a host cell system. With host cells come their own proteins. These host cell proteins (HCPs) constitute a major part of process-related impurities and can adversely affect drug safety, so it is critical that they are identified and quantified accurately.

A Fleet of Analyzers Keeps Work Flowing
An Interview with Timothy Sangster, Head of Bioanalysis and Immunology, Charles River Laboratories, Edinburgh

Delivering New Biologics to the Marketplace
Characterization and quantification of host cell proteins (HCPs) in biopharmaceutical development and manufacturing is a critical step to ensuring product safety. While this can be achieved using ELISA, mass spectrometry using the SCIEX TripleTOF® 6600 System is more specific and enables the identification and quantitation of each of the individual proteins present.
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.
Comprehensive Therapeutic Protein Characterization Using One Single Method
Get to know how CESI-MS will allow you to quickly and accurately characterize protein therapeutics for attributes in a single method by downloading this discovery kit.

What is your Method for Separating Challenging Polar Molecules?
From small ions like phosphate, herbicide degradation to metabolites, oligosaccharides, peptides, and proteins. How is your lab analyzing polar molecules? The reason I ask is there is a saying, if you have a charged or polar molecule, look to capillary electrophoresis (CE) first. While liquid chromatography (LC) is an ideal front-end separation tool for many types of molecules, as the following poster points out, “From Small to Very Large, Orthogonal, Sensitive Polar Molecule Analysis by CESI-MS,” there are some situations that call for CE over LC analysis. For those of you that are not familiar with CESI-MS, it is the combining of CE separation with electrospray ionization, into one dynamic process, within the same device.
The Power Behind LC-MS for Quantifying mAb Therapeutics
Quantitation of monoclonal antibodies (mAbs) in biological fluids is important during all stages of antibody drug development. First developed in the 1970s, therapeutic mAbs have both research and medicinal impact as they can be used for diagnosis and treatment of a wide variety of diseases, and have a high level of specificity.
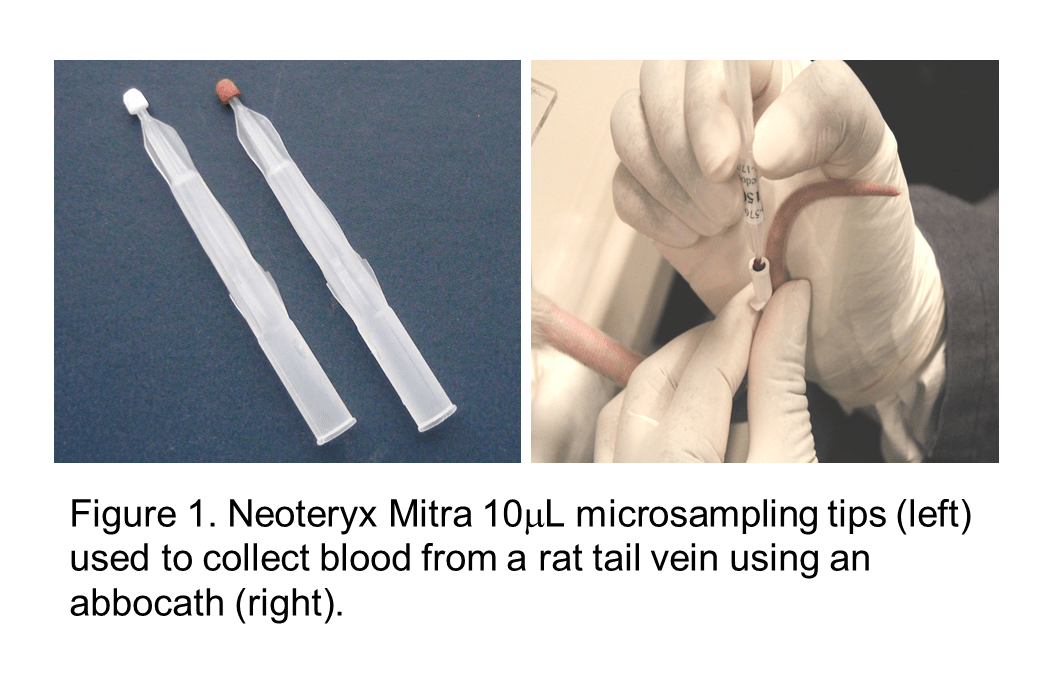
Volumetric Absorptive Microsampling and the SCIEX 6500+: A Pre-Clinical Case Study for the Biotherapeutic Exenatide
In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual
Say Goodbye to MetID Headaches, Say Hello to Automated Large Molecule Catabolism Processing
Is your mind swimming with large molecule catabolism data? Do you spend hours manually processing through complex spreadsheets to match your data with theoretical biologic catabolites? Are you wasting precious time by drawing out and processing your therapeutic peptide as a small molecule? Are you worried that you could be missing something?

Setting Records with Fast Glycan Technology
There is a lot of talk going around in the lab, and it has to do with the newly released Fast Glycan Labeling and Analysis technology. Where once research analysts needed to set aside days to perform glycan analysis, now takes an hour or so. Glycans are immediately identified by the software – so no need for data interpretation. That’s up to 5x faster than HILIC.

It’s a Point and Click World with the X500B QTOF System for Biologics Characterization
Did you know the X500B QTOF system makes point and click workflows for Biologics Characterization possible on your mass spectrometer? The newly-designed SCIEX OS software interface brings to life fluid navigation and ease of use so you can keep moving forward on your scientific discoveries. In fact, it’s so simple to learn and operate that you and your team can be up and running faster than you might expect.
See How Easy It Can Be to Get Expert Results for Biologics Characterization
Learning a new mass spec system can be a daunting task. Aside from the opportunity costs of training new users, you might face the hassle of downtime, and the wait to get expert help when needed. The X500B QTOF system puts a new spin on biologics characterization workflows because it is so easy to learn and operate that you can be up and running much faster than you expect. Powerful new software tools dramatically streamline method development and data processing, to enable everyone in your lab to get expert results. It’s fast because it’s easy, even for new users.

Discover the New X500B QTOF System, the Simpler, Faster Path to Biologics Characterization Answers
Have you ever wished for a compact instrument that delivers expert-level answers to your most complex biotherapeutic characterization challenges faster and easier than what you are doing now? At SCIEX, we recognize that even expert users want easier ways to perform daily characterization tasks and get great results every time. That’s why we set out to develop the X500B QTOF system: a robust and reliable new instrument and software solution that reduces complexity and simplifies biologics characterization workflows so every scientist can get expert-level results
How to Achieve Higher Sensitivity with Hybrid Immunoaffinity LC-MS Assays
Protein-based biotherapeutics, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) are a growing component of pharmaceutical companies’ drug pipelines. The growth of ADCs in particular is due to their ability to selectivity target and deliver a potent molecule to a cancer cell based on a specific tumor marker. In order to support this growing class of new drug molecules, robust and reliable bioanalytical methods are required. While ligand binding assays (LBAs) like ELISA have been the most popular platform for biotherapeutic quantitation, bioanalytical scientists have been increasingly adopting hybrid LBA/LC-MS methods in this area.

The Application of Research Grade MetabolitePilot™ Software for the Determination of the Catabolic Peptide Products of Exenatide
The stability of peptide and protein biotherapeutics directly impacts their pharmacokinetic profile, efficacy, and safety. It is therefore essential to characterize the stability of a given bio-therapeutic including both in-vivo and in-vitro catabolism, thereby...

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.