For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.
Tags

The whys behind the dos and don’ts of oligonucleotide analysis
We know that LC-MS oligonucleotide analysis can have its share of challenges—challenges with sensitivity, challenges with adduct formation and challenges with data analysis, to name just a few. That’s why this blog takes a closer look at the dos and don’ts of this type of analysis and explores some keys to success. It also explains why following these simple rules can vastly improve your oligonucleotide characterization and quantitation efficiency and success.
Top 7 Echo® MS system customer questions—answered
You asked, we answered! With analysis speeds of at least 1 sample per second, the Echo® MS system has created a buzz in the industry. This is up to 50x faster than conventional LC-MS/MS. This revolutionary tool for drug discovery and development has led to...

An interview from the Science Explorer about the Echo® MS system
The Science Explorer interviews Neil Walsh from SCIEX to discuss the significant application areas of the Echo® MS System and what makes this system so attractive to biopharmaceutical laboratories.

The Echo® MS system: Is it reproducible? Yes… yes… yes!
The Echo® MS System is an exciting new platform that dramatically speeds sample analysis for quantitative MS studies. Because of its unique and innovative technology, the system can analyze samples faster than ever before—but without the need for LC.

Scale it up! The Echo® MS System delivers unprecedented levels of productivity
Imagine the productivity gains your lab could achieve with a technology that not only analyzes samples up to 50x faster than conventional quantitative LC-MS, but also eliminates tedious sample preparation, time-consuming LC method development and chromatographic run...

How fast is fast? The Echo® MS System sets the record
How fast is fast? Cheetahs. Usain Bolt. Tachyons. The Echo® MS system. What do these things have in common? They’re all fast. REALLY fast. In fact, they’re the fastest in their categories: the fastest land mammal, the fastest human sprinter, the fastest subatomic...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.

Ever thought of breaking the high-throughput sound barrier for drug discovery?
Wouldn’t it be great if we really could “get time back” or even “buy time”? When developing pharmaceuticals, it takes years to bring a new therapy to the market due to the linear nature of the process. As the saying goes, “Time waits for no one.” But what if we could...
The Power Behind LC-MS for Quantifying mAb Therapeutics
Quantitation of monoclonal antibodies (mAbs) in biological fluids is important during all stages of antibody drug development. First developed in the 1970s, therapeutic mAbs have both research and medicinal impact as they can be used for diagnosis and treatment of a wide variety of diseases, and have a high level of specificity.
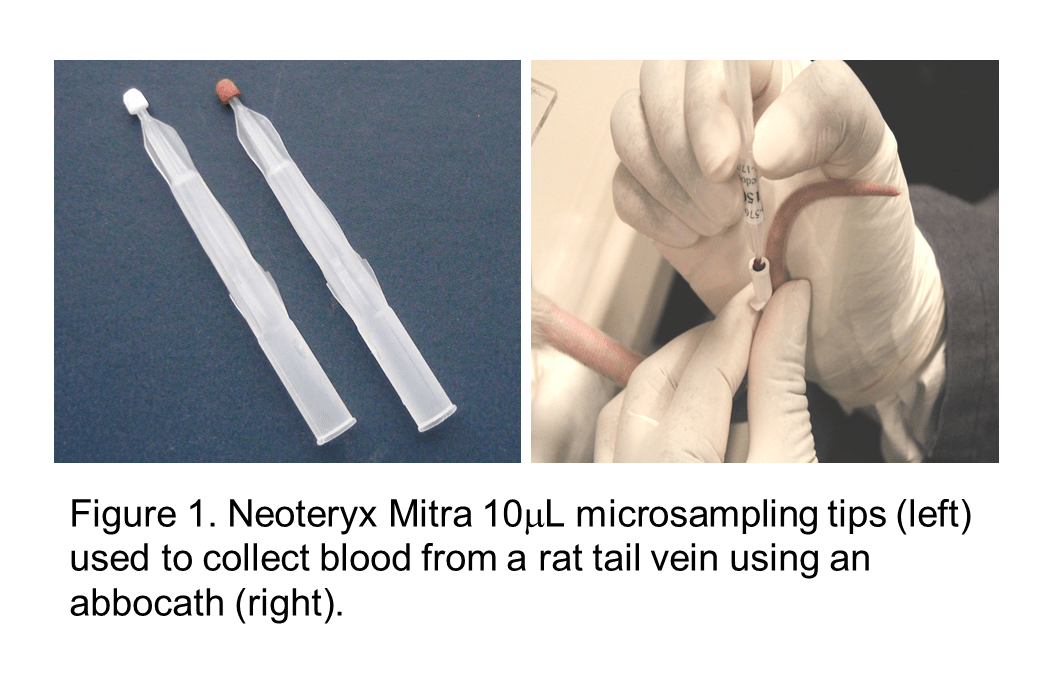
Volumetric Absorptive Microsampling and the SCIEX 6500+: A Pre-Clinical Case Study for the Biotherapeutic Exenatide
In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual
Say Goodbye to MetID Headaches, Say Hello to Automated Large Molecule Catabolism Processing
Is your mind swimming with large molecule catabolism data? Do you spend hours manually processing through complex spreadsheets to match your data with theoretical biologic catabolites? Are you wasting precious time by drawing out and processing your therapeutic peptide as a small molecule? Are you worried that you could be missing something?

Setting Records with Fast Glycan Technology
There is a lot of talk going around in the lab, and it has to do with the newly released Fast Glycan Labeling and Analysis technology. Where once research analysts needed to set aside days to perform glycan analysis, now takes an hour or so. Glycans are immediately identified by the software – so no need for data interpretation. That’s up to 5x faster than HILIC.

It’s a Point and Click World with the X500B QTOF System for Biologics Characterization
Did you know the X500B QTOF system makes point and click workflows for Biologics Characterization possible on your mass spectrometer? The newly-designed SCIEX OS software interface brings to life fluid navigation and ease of use so you can keep moving forward on your scientific discoveries. In fact, it’s so simple to learn and operate that you and your team can be up and running faster than you might expect.
See How Easy It Can Be to Get Expert Results for Biologics Characterization
Learning a new mass spec system can be a daunting task. Aside from the opportunity costs of training new users, you might face the hassle of downtime, and the wait to get expert help when needed. The X500B QTOF system puts a new spin on biologics characterization workflows because it is so easy to learn and operate that you can be up and running much faster than you expect. Powerful new software tools dramatically streamline method development and data processing, to enable everyone in your lab to get expert results. It’s fast because it’s easy, even for new users.

Discover the New X500B QTOF System, the Simpler, Faster Path to Biologics Characterization Answers
Have you ever wished for a compact instrument that delivers expert-level answers to your most complex biotherapeutic characterization challenges faster and easier than what you are doing now? At SCIEX, we recognize that even expert users want easier ways to perform daily characterization tasks and get great results every time. That’s why we set out to develop the X500B QTOF system: a robust and reliable new instrument and software solution that reduces complexity and simplifies biologics characterization workflows so every scientist can get expert-level results
How to Achieve Higher Sensitivity with Hybrid Immunoaffinity LC-MS Assays
Protein-based biotherapeutics, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) are a growing component of pharmaceutical companies’ drug pipelines. The growth of ADCs in particular is due to their ability to selectivity target and deliver a potent molecule to a cancer cell based on a specific tumor marker. In order to support this growing class of new drug molecules, robust and reliable bioanalytical methods are required. While ligand binding assays (LBAs) like ELISA have been the most popular platform for biotherapeutic quantitation, bioanalytical scientists have been increasingly adopting hybrid LBA/LC-MS methods in this area.

The Application of Research Grade MetabolitePilot™ Software for the Determination of the Catabolic Peptide Products of Exenatide
The stability of peptide and protein biotherapeutics directly impacts their pharmacokinetic profile, efficacy, and safety. It is therefore essential to characterize the stability of a given bio-therapeutic including both in-vivo and in-vitro catabolism, thereby...

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...
Harnessing the Power of MRM3 for Large Molecule Quantitative Bioanalysis
In a previous blog outlining the advantages of high-resolution accurate mass measurements for protein quantitation using the TripleTOF 6600, it was noted that although the triple-stage quadrupole demonstrated high sensitivity when operated in multiple reaction monitoring mode (MRM), the relatively low-resolution measurement of m/z failed to discriminate Rituximab response from nominally isobaric interferences given the complexity of the proteolytically digested samples (June 28/2016). While the accurate mass filtering capabilities of the TripleTOF 6600 represents one mechanism for achieving increased selectivity over MRM, the triple quadrupole/linear ion trap (LIT) hybrid platform represented by the QTRAP® 4500, 5500, 6500 and 6500+ systems provides an alternative technique by leveraging a third stage of MS, often referred to as MRM3. In this blog, we outline the MRM3 scan function and survey several large molecule applications which utilize the additional stage of fragmentation in the LIT to yield significant improvements in achievable detection limits when compared to MRM.
See What More You Can do With 360 Degree Biologics Characterization
Ever wish you had your own team of mass spectrometry experts at your side when working through biologics development and characterization challenges? With SCIEX 360° Innovations complex biologics characterization is streamlined with a full suite of mass spectrometry (MS), capillary electrophoresis (CE) systems, software, and services from SCIEX experts.

You’ve Seen It… Now Try It! BioPharmaView Software 2.0
At ASMS this year, the newest version of BioPharmaView Software was released. This software simplifies the processing of biotherapeutic data for characterization and comparability which can dramatically improve your productivity. BioPharmaView 2.0 Software accelerates characterization and comparability studies and simplifies reporting, so you can make better decisions, faster.

Three Infographics to Show You How to Overcome Challenges in Transitioning to Biologics Bioanalysis
The move to large molecules in Pharma is accelerating, offering unprecedented opportunities to improve human health and expand into new markets. But for those with extensive experience with small molecule bioanalysis, the shift to biologics can be challenging, from Sample Prep to Instrumentation and Software, to Methods and Training:

Rapid Characterization of Biologics using CESI-MS
Today, 30 monoclonal antibodies (mAbs), have been approved for the treatment of certain cancers, autoimmune and infectious diseases. Even more are in development, and perhaps you and your team of scientists are working on one now. Keeping pace with fast development timelines while performing comprehensive characterization of biologic candidates can be challenging. However, more and more, scientists are tackling these challenges with new techniques to speed and simplify their characterization workflows. Read more in the application note, “Rapid Characterization of Biologics using a CESI 8000 – SCIEX TripleTOF® System,” found in the Biologics Analytical Characterization Compendium, which highlights how CESI separation coupled with high-resolution mass spectrometry can provide a comprehensive characterization of biotherapeutics.
Protein Quantitation Workflows using the TripleTOF 6600: A Case Study for Rituximab
Although the triple-stage quadrupole (QQQ) mass spectrometer remains the pillar for quantitative LC-MS/MS bioanalytical assays, due in part to the platforms’ high duty cycle when operated in multiple-reaction monitoring (MRM) mode, the applicability of high-resolution mass spectrometry (HRMS) has become of increasing importance for protein quantitation given the complexity of proteolytically digested samples in the surrogate peptide approach. While the QQQ demonstrates high sensitivity and specificity, the relatively low-resolution measurement of m/z may fail to differentiate analyte response from nominally isobaric background interference. In contrast, HRMS with accurate mass assignment of product ion allows interference to be resolved through judicious selection of a post-acquisition mass extraction window whose tolerance is largely dictated by the effective resolution and stability of mass calibration.

Fast, Efficient, Disulfide Bond Mapping Using BioPharmaView™ Software
Fast LC-MS acquisition and automated data processing will help you speed up peptide mapping of your biotherapeutic, including critical disulfide bond and post-translational modification characterization. SCIEX helps you untangle the complexity of disulfide bonds, speeding up your characterization process.

Characterize and Monitor Host Cell Proteins (HCPs) Using SWATH Acquisition Technology
During drug development, the removal of impurities and purification of a final drug product is absolutely essential in order to ensure the safety and efficacy of a therapeutic drug. Of particular concern for biologics are impurities that can stem from host cell proteins. Because biologics are developed through cell culture and fermentation within a host cell, proteins from this host cell can be co-purified with the final biologic. These host cell proteins or HCPs can cause the final product to have undesired side-effects such as eliciting an immune response in patients taking the drug, or affecting the drug’s stability or efficacy. As a result, regulating agencies require drug companies to monitor levels of HCPs during the development and purification of a biologic and to remove HCPs to an acceptable level in the final biotherapeutic product.