Once your data processing sessions have completed, the results files are saved back to either SCIEX Data Store or BaseSpace. These can be downloaded from the cloud to your desktop for additional analysis. Please see these community posts to learn more: Explaining the...
Tags
PFAS testing: 2024 in review and what to expect for 2025
For as long as PFAS persist in the environment, there is no doubt they will persist in our conversations as environmental scientists. Globally, PFAS contamination has been detected in water supplies, soil and even in the blood of people and wildlife. Different countries are at various stages of addressing PFAS contamination and many governments have set regulatory limits and are working on assessing the extent of contamination, cleaning up affected sites and researching safer alternatives.

Inside the box: Acoustic ejection mass spectrometry for drug discovery
On average, it takes 10-15 years and 1-2 billion dollars to approve a new pharmaceutical for clinical use. Since approximately 90% of new drug candidates fail in clinical development, the ability to make early, informed and accurate decisions on the safety and efficacy of new hits and leads is key to increasing the chances of success.

Unveiling the power of ZT Scan DIA: Insights from Ludwig Sinn’s presentation at World HUPO Congress 2024
In a recent presentation at the World HUPO Congress 2024, Ludwig Sinn from the Ralser lab shared exciting advancements in proteomics research, focusing on the innovative ZT Scan DIA acquisition modes developed in collaboration with SCIEX. Let us explore the key highlights and benefits of this innovative technology.

Exploring the power of ZT Scan DIA
In a recent presentation, Tim Heymann from Mann Lab at the Max Planck Institute of Biochemistry shared his first impressions of ZT Scan DIA, the novel data-independent acquisition strategy from SCIEX, highlighting its innovative approach and significant benefits for proteomics research. Let’s dive into the key points from his insightful talk.

Inside the box: Complementary fragmentation with LC-MS for Metabolite Identification
Liquid chromatography-mass spectrometry is commonly used for Met ID but confident soft spot identification is not always possible. Imagine the advantage of unambiguous metabolite identification using liquid chromatography-mass spectrometry (LC-MS) reducing the need for additional safety testing during drug discovery. Quickly and easily generate the information you need using routine assays that are robust and efficient, enabling confident decision-making while also saving time and money.

Tips and tricks from our application experts: AAV analysis with CE
Peter Holper, Staff Applications Scientist at SCIEX, US, shares his tips and tricks on AAV analysis using CE with the BioPhase 8800 system and the PA 800 Plus system.
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

Sensitivity, It’s at the Very Heart of Who We Are
Walk into any modern pharmaceutical company these days, and you’ll likely find at least one if not many, SCIEX LC-MS/MS instruments. Assays for the detection and quantitation of small molecule drugs, metabolites, biotherapeutics, biomarkers, and many other analytes...

The Advantages of a Software Maintenance Agreement
You’ve bought your mass spectrometer. You’ve installed all your hardware. You’ve got your operating software set up. You’ve mastered your software workflow modules to optimize quantitative and qualitative data processing. That’s it, right? Even though your system is...

You’ve Edited the Gene. Now What?
Protein-Level Verification Without The Need For Antibodies Using SWATH® Acquisition. Fast, Comprehensive, and Highly Reproducible Although humans have been able to influence the traits of plants and animals for thousands of years through domestication and selective...

Environmental Scientists: Here’s Why You Should Consider Nominal Mass Spectrometry for Your Routine PFAS and Gen-X Testing in Drinking Water
How are you feeling about PFAS regulation right now? If you’re like us, you probably find it hard to pinpoint one specific feeling. But if we took a look at the current state of affairs, we’d all probably agree that it’s a minefield of uncertainty. Under the Safe...
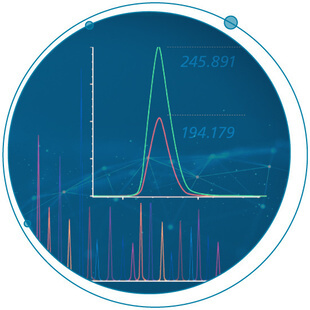
Why Quant?
Every decision you make requires a number As a pharmaceutical scientist, you understand the challenges in getting a new drug through the discovery and development pipeline. As an innovation partner in mass spectrometry, SCIEX works continuously with you, our...

6 Tips to Keep Your Lab’s Software Up and Running
6 Tips to Keep Your Lab’s Software Up and Running Is your lab utilizing SCIEX instrumentation but hesitant about upgrading to the latest software release? Perhaps you are uncertain about key features, error codes, or output. Not to worry, behind every great mass...
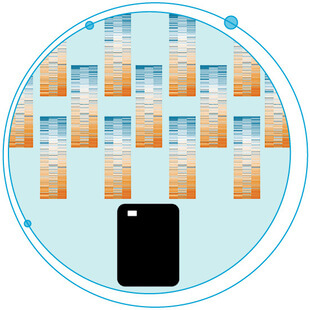
A Smart Way to Profit from the Wealth of Biobanks
Microflow LC with SWATH® Acquisition for Digitizing Biobanks What if you could access thousands of high-quality samples for your research? What if these samples were well-annotated biological specimens? And what if they were carefully segmented into just the...
Why Conventional Flow LC-MS/MS Bioanalysis Is out and Microflow Is In
New technologies can transform a laboratory’s throughput and efficiency. At Alturas, if we try out a new technology, we ask: Does it work? Is it convenient? Is it rugged? Are we getting good results? When we look at the convenience, uptime, and overall data that we’re...

Food Testing Labs: What Technology Is Right for You?
Consumer safety is the driving force behind food analysis. From field (or ocean) to fork, there are numerous opportunities for food to become potentially hazardous to human health. As fast as new contaminants enter the food chain, changes in regulation and legislation...

Fueling Up: Eating To Stay Strong When You Have Cancer
This is the third and final post in our series in honor of International Women’s Day and our collaboration with World Cancer Research Fund. To wrap up, Deborah Howland talks about the importance of diet for anyone facing cancer – or trying to prevent it. A specialist...

5 Demands of Forensic Toxicologists and the Ultra-Fast Method That Meets Them
There just isn’t a screening approach available that can give us everything we need when throughput is the priority. Sound familiar? For many toxicology labs, there is a misconception that compromises need to be made when it comes to high throughput drug screening....

Uncovering the Links Between Childhood Growth, Body Size, and a Woman’s Risk of Breast Cancer
Welcome to the second in a series of posts marking International Women’s Day, and our ongoing support of World Cancer Research Fund. This installment is a review by Dr. Jennifer Baker, of her work, that, with the help of a WCRF grant, is studying body size and its links to breast cancer. Dr. Baker, Lead Investigator at Denmark’s Frederiksberg Hospital, has a Ph.D. in Human Nutrition from Cornell University. Her research focuses on clinical epidemiology.

Global Trends That Will Affect Neonicotinoid Usage in 2019
Neonicotinoids have become the most widely used class of insecticide in the world, with registration in 120 countries. However, these pesticides have become embroiled in multi-year controversy in Europe and North America due to their risk to beneficial...

A Multi-Omics Database for Tomato Research and Breeding
Ace. Beefsteak. Big Boy. Kumato. Early Girl. Roma. Sun Gold. San Marzano. These are just a few of the thousands of varieties of tomato plants available today. And while all of these varieties may be very different with respect to crop yield, disease resistance,...

A Diagnosis And A “Difficult Waiting Game”
We are thrilled to mark International Women’s Day 2019 by making our fourth annual donation to World Cancer Research Fund, a global not-for-profit organization and leading authority on the links between diet, nutrition, physical activity, and cancer. In fact, our...

Phthalates: The Everywhere Chemical That’s Hard to See, Not Anymore with LC-MS/MS
A few months back, the American Academy of Pediatrics published a technical report on the use of chemicals in food processing and the negative health effects on children. One of the main culprits is phthalates. The 411 of PhthalatesPhthalates are esters of phthalic...

The SCIEX Software Roadmap – The Journey Towards Windows 10 Compatibility is Complete
At SCIEX we are committed to providing you with the most up-to-date tools and technologies to meet the evolving needs of the laboratory. As part of that commitment, we were introducing our software product roadmap towards Windows 10 compatibility back in 2017. Many of...
10 Minutes in One Shot. That’s How Quickly You Can Screen 664 Forensics Compounds
Drug testing is a moving target. As novel psychoactive substances (NPS) rapidly emerge as a new class of designer stimulants (DS), global use has reached an all-time high over the last decade. Supposedly ‘legal’ alternatives to internationally controlled drugs, these...
Turbocharge THC-COOH Hair Analysis with Super Sensitive LC-MS/MS and Solid Phase Extraction
Do you want a more efficient workflow for the forensic analysis of THC-COOH in hair samples? Yes! Do we know a simple, highly sensitive technique? You bet! Read on to find out how you can detect THC-COOH in hair down to 0.2 pg/mg trace concentration levels with...
Fast Novel Psychoactive Substance Testing, At Your Fingertips
Why Dried Blood Spot Analysis Is the Way to Go for Novel Psychoactive Substances(And Why You Need This SCIEX LC-MS/MS Method) Anyone with children will remember that moment in the hospital when the nurse pricks the heel of your tiny new baby to squeeze out a few drops...