This is the third and final post in our series in honor of International Women’s Day and our collaboration with World Cancer Research Fund. To wrap up, Deborah Howland talks about the importance of diet for anyone facing cancer – or trying to prevent it. A specialist...
Tags
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.
Protein Quantitation Workflows using the TripleTOF 6600: A Case Study for Rituximab
Although the triple-stage quadrupole (QQQ) mass spectrometer remains the pillar for quantitative LC-MS/MS bioanalytical assays, due in part to the platforms’ high duty cycle when operated in multiple-reaction monitoring (MRM) mode, the applicability of high-resolution mass spectrometry (HRMS) has become of increasing importance for protein quantitation given the complexity of proteolytically digested samples in the surrogate peptide approach. While the QQQ demonstrates high sensitivity and specificity, the relatively low-resolution measurement of m/z may fail to differentiate analyte response from nominally isobaric background interference. In contrast, HRMS with accurate mass assignment of product ion allows interference to be resolved through judicious selection of a post-acquisition mass extraction window whose tolerance is largely dictated by the effective resolution and stability of mass calibration.

Rise of the Super Bugs
The term “antibiotic-free” is becoming more and more popular in food advertising these days. Take Subway for example; in March the company elevated their antibiotic-free policy and introduced a new antibiotic-free rotisserie-style chicken sub, and they plan to, “Nix antibiotics in all its meat by 2025.”

Glyphosate, a Polar Pest Put to Test
No other pesticide has courted more media attention and controversy in recent months than glyphosate, with governments and national agencies debating its use and health effects.

Using Mass Spec to Detect Trace Explosives
The importance of protecting a country’s border is a very topical issue. The war on drugs and terror is a 24/7 task 366 days a year (2016 is a leap year). The government agencies in charge must be vigilant and maintain instrumentation to prevent terrorism, drug trafficking, and other illegal activities. Mass Spectrometry is rapidly becoming the instrument of choice for border agencies throughout the world when it comes to explosive trace detection and forensic drug compounds.

Routine Food Testing Using Mass Spectrometry
These days, it is not uncommon to hear about the overzealous application of pesticides to crops or the injection of antibiotics into animals. From grocery stores to restaurants, our food is at risk. How then, can consumers be assured that chemical contaminants like these , not to mention the risk of mycotoxin compounds are not making their way to your dinner table?
Industrialize Your Quantitative Proteomics Using a More Simplified Sample Prep
in part 1 and part 2 of this blog series we discussed how you can increase your efficiency for high throughput quantitative proteomics by industrializing your sample analysis and data processing. Microflow SWATH® Acquisition on your TripleTOF® system coupled with OneOmics™ data analysis tools allow you to run samples faster, collect data faster, and process your data files faster. It all adds up to getting more meaningful biological information in a shorter amount of time.

Quantify and Identify Pesticides in Complex Food Samples Using the QTRAP 6500 LC-MS/MS System
Recent regulations on food analysis require screening for pesticides using confirmatory techniques, such as GC-MS and LC-MS/MS. More than 1000 pesticides are used worldwide and, along with their metabolites and degradation products, are present in food. There is a demand for powerful and rapid analytical methods that can identify pesticides with high confidence in a broad range of food matrices and quantify at low concentrations with good accuracy and reproducibility. Challenges for pesticide residue laboratories at the moment are the request to test for more compounds, in a wider range of samples, all without sacrificing data quality.

Industrialize Your Quantitative Proteomics with the OneOmics Project
For many labs, the days are long gone when it was acceptable to run only a few samples a week for your quantitative proteomics projects. The pressure for faster turn-around times, to support larger cohort studies, to sustain multiple research directions, and to transition from a purely unbiased discovery mode to verifying something truly unique and interesting, all demand a faster pace. Many labs are now being asked to analyze a hundred samples a week or more. In part 1 of this blog series, we saw how moving to a microflow SWATH workflow can dramatically increase your throughput with little compromise on overall results. In this part, we’ll address what to do with all of this data because it’s just no good if all we’ve done is move the bottleneck downstream.

Fast, Efficient, Disulfide Bond Mapping Using BioPharmaView™ Software
Fast LC-MS acquisition and automated data processing will help you speed up peptide mapping of your biotherapeutic, including critical disulfide bond and post-translational modification characterization. SCIEX helps you untangle the complexity of disulfide bonds, speeding up your characterization process.
Bottom-Up Proteomics: A Discussion with Christie Hunter
Biocompare recently featured an article on Bottom-Up Proteomics. I had a chance to follow up with Christie Hunter and expand on some of the questions featured in the article:

Characterize and Monitor Host Cell Proteins (HCPs) Using SWATH Acquisition Technology
During drug development, the removal of impurities and purification of a final drug product is absolutely essential in order to ensure the safety and efficacy of a therapeutic drug. Of particular concern for biologics are impurities that can stem from host cell proteins. Because biologics are developed through cell culture and fermentation within a host cell, proteins from this host cell can be co-purified with the final biologic. These host cell proteins or HCPs can cause the final product to have undesired side-effects such as eliciting an immune response in patients taking the drug, or affecting the drug’s stability or efficacy. As a result, regulating agencies require drug companies to monitor levels of HCPs during the development and purification of a biologic and to remove HCPs to an acceptable level in the final biotherapeutic product.
Simplifying Biologics Bioanalysis Sample Prep
These days, everyone seems to be furiously scratching tickets to become instant winners, but I’ll bet you didn’t expect to find sample prep tips that way. For large molecule bioanalysis, preparing your samples can be one of the biggest challenges. It’s a whole different world from traditional small molecule bioanalysis. SCIEX has developed techniques and automation that make biologics sample prep simpler and faster, with reproducible results.
Taking on Precision Medicine with Industrialized Proteomics
What if we could deliver the right treatment at the right time, to the right person to better, more effectively treat complex disease? This is the promise of precision medicine, to be able to approach complex disease treatment and prevention by taking into account individual variability in genes, environment, and lifestyle for each person.

The Connection Between Mass Spectrometry and Space Exploration
Mass spectrometry has been used for some pretty fascinating applications in our world – like testing for steroid use in athletes1, measuring pesticides in grapes2, assessing the efficiency of a psoriasis drug3, and whether that expensive bottle of 100% olive oil is, well, really 100% olive oil.4 But did you know mass spec is also used out of this world? Like… in space?
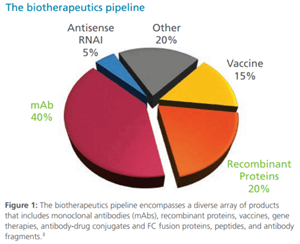
The Future of Biologics Drug Development is Today
Since the 1982 approval of Eli Lilly’s recombinant human insulin, Humulin, biotherapeutic drug development has steadily grown into a global market valued at $140 billion in 2013, increased from $25 billion in 2001
A Hybrid LBA/LC-MS Assay – Your Questions Answered
Last week we posted a blog on Biologics Bioanalysis Key Challenges, where we presented a webinar on those key challenges.
Using Mass Spectrometry to Identify and Quantify Contaminants in Water Samples
Access to clean wholesome water is one of our basic human rights. Human engineering has designed incredible methods to collect, filter, purify, store and distribute water to billions of us worldwide, but does this mean that our water is completely safe to drink? Also,...

LC-MS/MS Method for Biotherapeutic Drug Development Challenges
Traditionally, the pharmacokinetic profile of biotherapeutics such as insulin glargine, adalimumab, trastuzumab and others, used gold standard LBAs to assess dose-response during drug discovery and development. However, LBAs require a specific antibody reagent to be developed for each mAb variant, a process that is often incompatible with the compressed timeframes encountered during the initial stages of drug development.

QTOF Technology for Targeted and Unknown Forensic Drugs Screening Workflows
In this study, the Wisconsin State Laboratory of Hygiene (WSLH) outlines the comparison of their existing technology and how SCIEX LC-MS/MS systems can assist them in their forensic research. The WSLH routinely analyze for 300 forensic drug compounds in over 18,000 samples per year.

Expert Advice to Help You with Routine Food Testing in the Lab
Between 3-6 November 2015, the Recent Advances in Food Analysis (RAFA) 2015 Symposium took place in Prague, Czech Republic.