Each year, the Drug Enforcement Administration (DEA) Special Testing and Research Laboratory publishes an Emerging Threat Report. The data contained in the report is updated quarterly and represents a snapshot of the drug evidence seized and analyzed by the DEA in the...
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.

Metabolite identification and peace of mind
Managing metabolite identification (Met ID) studies is challenging, so what is at the top of your priority list as you plan the year ahead? Ensuring you have the data needed to manage product safety, meeting deadlines, staff recruitment and training, maintaining compliance, capital expenses, or something else?

What has the Echo® MS system done for the pharma industry? (And don’t just take our word for it!)
SCIEX was very proud to have an illustration of the Acoustic Ejection Mass Spectrometry (AEMS) technology that powers the Echo® MS system on the front cover of the Journal of the American Society for Mass Spectrometry in January 2023. The associated article—Ultrahigh-Throughput Intact Protein Analysis with Acoustic Ejection Mass Spectrometry—was co-authored by scientists from SCIEX and Merck.

New features in OneOmics suite
I just wanted to thank the readers here, both the OneOmics suite users who’ve shared their time and watched OneOmics grow, and for all the talented developers and scientists who’ve made OneOmics suite what it is today.

SCIEX Lands HUPO Science and Technology Award
We are pleased to congratulate its research scientists Stephen Tate and Ron Bonner (retired) for being awarded this year’s Science and Technology award at HUPO 2017 in Dublin Ireland. The Science and Technology Award at HUPO recognizes an individual or team who were key in the commercialization of a technology, product, or procedure that advances proteomics research

Happy Birthday to SWATH Acquisition! 5 Years of Innovation
With its introduction at the HUPO World Congress in 2010 in Sydney Australia by Ruedi Aebersold, SWATH® Acquisition instantly intrigued scientists around the world. Here was a new technique with the potential to revolutionize the way proteomics studies were performed! Based on a data independent acquisition strategy using a SCIEX TripleTOF® 5600 system, SWATH was able to consistently identify and quantify at least as many peptides and proteins as other far more mature proteomics strategies on the market, but with quantitative accuracy and reproducibility rivaling gold standard MRM experiments! This solution was made broadly available to researchers with a full launch of SWATH Acquisition in the Analyst® TF 1.6 Software on the TripleTOF 5600+ System at ASMS 2012 in Vancouver (A Mine of Quantitative Proteomic Information. Prof Dr. Ruedi Aebersold, Head of the Department of Biology, ETH Zurich).

5 Tips for Calibrating a QTOF Mass Spectrometer
Do you have questions about your mass spec? How about a workflow? Our community members are involved in active discussions and receive expert answers from customers like you, SCIEX scientists, and support specialists every week. One recent topic concerned the automatic calibration on TripleTOF® systems as answered by Dr. Christie Hunter whose focus is developing and testing innovative MS workflows for omics research through working collaboratively with the instrument, chemistry, and software research groups.
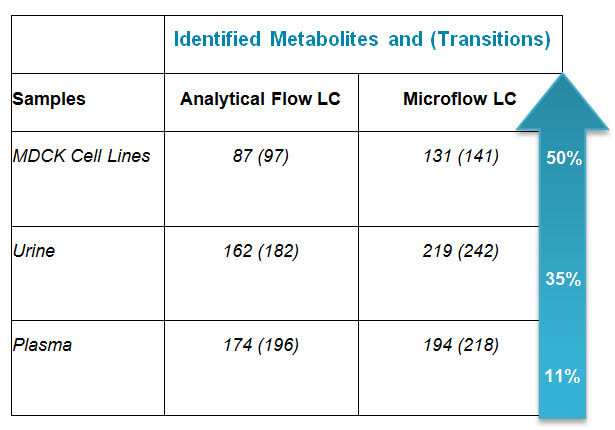
Why Microflow HILIC Chromatography for Targeted Metabolomics Applications?
I recently had the opportunity to catch up with Baljit Ubhi to discuss the top questions you’re asking in regards to using Microflow HILIIC Chromatography for Targeted Metabolomics. Here’s what Bal said:

Discover The Benefits of Knowledge Base Articles
Did you know you can access Knowledge Base Articles for trending user questions compiled and answered by SCIEX support experts? Doing so may help to reduce your support calls, not to mention downtime. Instead of waiting for a problem to occur, you can stay on top of it, and be a part of the solution. To give you an idea of trending articles, consider the how this past month saw questions and answers including:

How Easy Is It to Relocate a Mass Spectrometer?
When you’re in the process of moving your lab, across the corridor or to another country, there’s a lot to think about. Adding to the stress, there’s not always a lot of time to plan, or budget allocated for the process, especially in the case of unexpected urgent maintenance work.

SWATH Acquisition – Master of All Trades
SWATH® Acquisition is an innovative strategy for acquiring data on a TripleTOF® mass spectrometer. In a previous blog, we learned how SWATH works. Now let’s learn what it can do for different applications:

Data Independent Acquisition Mass Spectrometry with the Power of SWATH
There are many different methods in use today to acquire data on a mass spectrometer, but few have generated as much buzz in recent years as SWATH technology. First reported 5 years ago by Ruedi Aebersold and his group1, SWATH® Acquisition on a TripleTOF® instrument has rapidly become one of the premier acquisition strategies for identification and quantitation of complex samples. But what exactly is SWATH and why is it so powerful? In order to answer these questions, let’s first take a step back and look at the larger picture.
Vice President Biden Announces Agreement Naming Children’s Medical Research Institute’s ProCan Lab to the ‘Cancer Moonshot’ Initiative
A key goal of the ‘Cancer Moonshot’ initiative is the advancement of precision medicine, with the goal of making more targeted therapies available to more cancer patients. And researchers believe that the time is right, with the new technological innovations, the new insight into the biology of cancer and big improvements in the handling of ‘big data.’

Stoller Biomarker Discovery Centre, Addressing Some of the Biggest Issues in Medicine
The Stoller Biomarker Discovery Center, developed in partnership with SCIEX, was created to develop new omics technologies for biomarker research to understand the root cause of diseases such as cancer, cardiovascular disease, and autoimmune diseases. We initially announced our collaboration with the University of Manchester back in October 2015.
The History of Isotopic Labels for Quantitative Proteomics
Proteomics has become a vital tool for biological scientists performing research on the healthy and diseased states of living things. It involves the large scale and systematic analysis of all proteins within a given cell, tissue, or organism. Because proteins are regulated by many different internal and external stimuli, the proteome is dynamic and quantities of proteins can change from one state to the next. Therefore, in order to be of the highest utility, proteomics experiments need to both identify and quantify proteins so that comparative studies can be done, such as between healthy cells and tumor cells, or the comparison of different treatment regimens.

Taking care of your mass spectrometer—Onsite troubleshooting and maintenance training for today’s lab
Recently, we asked customers to tell us about their biggest challenges so we could customize training programs to meet the needs of today’s growing lab. Without hesitation, most of you said uptime and employee training are your most critical needs. As a result, our...
The Promise of Precision Medicine
Here is the latest update on the Worldwide Efforts to Accelerate Precision Medicine
The NIH recently issued a press release in early July announcing $55 million in awards. According to the release, the $55 million award in the fiscal year 2016 will go towards building the foundational partnerships and infrastructure needed to launch the Cohort Program of President Obama’s Precision Medicine Initiative (PMI). The PMI Cohort Program is a landmark longitudinal research effort that aims to engage 1 million or more U.S. participants to improve the ability to prevent and treat disease based on individual differences in lifestyle, environment, and genetics.
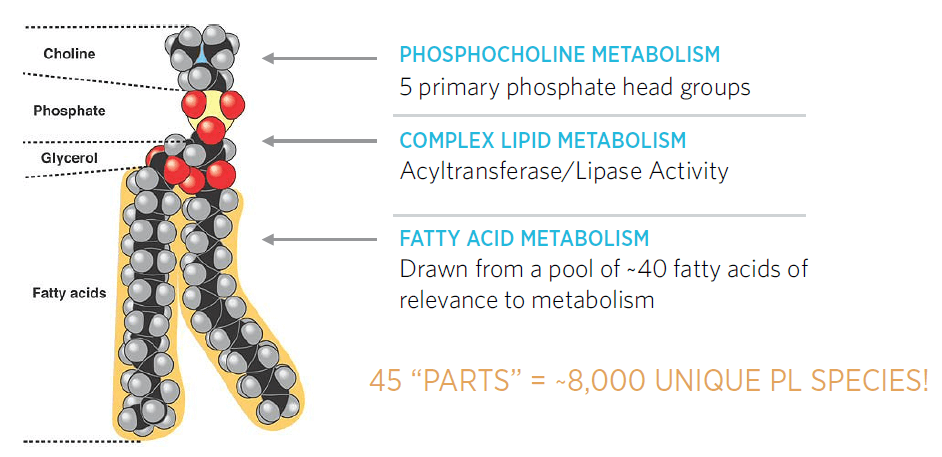
Why Study Lipids?
I had an opportunity to follow up with Steven M Watkins, Ph.D. to talk about the importance of studying lipids in disease. Steve has been working in the lipids field for over 20 years and is one of the foremost experts in lipid biology. Steve founded Lipomics in 2000, an early metabolomics company focused on quantitative lipidomics and had followed that company through a series of changes that led to its involvement in the clinical diagnostic development and global metabolomics. Steve authored over 70 peer-reviewed publications including several book chapters on lipids and lipid metabolism. His presentations on this topic are fascinating and very informative, so I wanted to capture some of his thinking here!

Improved complex sample processing for higher quality of results, reproducibility and depth of proteomic analysis
SCIEX partners to improve depth of proteome coverage
SCIEX and Pressure BioSciences address a major challenge for researchers performing complex sample preparation by marketing a complete solution to increase the depth, breadth, and reproducibility of protein extraction, digestion, and quantitation in all tissue types, especially challenging samples like tumors.
Industrialize Your Quantitative Proteomics Using a More Simplified Sample Prep
in part 1 and part 2 of this blog series we discussed how you can increase your efficiency for high throughput quantitative proteomics by industrializing your sample analysis and data processing. Microflow SWATH® Acquisition on your TripleTOF® system coupled with OneOmics™ data analysis tools allow you to run samples faster, collect data faster, and process your data files faster. It all adds up to getting more meaningful biological information in a shorter amount of time.

Industrialize Your Quantitative Proteomics with the OneOmics Project
For many labs, the days are long gone when it was acceptable to run only a few samples a week for your quantitative proteomics projects. The pressure for faster turn-around times, to support larger cohort studies, to sustain multiple research directions, and to transition from a purely unbiased discovery mode to verifying something truly unique and interesting, all demand a faster pace. Many labs are now being asked to analyze a hundred samples a week or more. In part 1 of this blog series, we saw how moving to a microflow SWATH workflow can dramatically increase your throughput with little compromise on overall results. In this part, we’ll address what to do with all of this data because it’s just no good if all we’ve done is move the bottleneck downstream.
Taking on Precision Medicine with Industrialized Proteomics
What if we could deliver the right treatment at the right time, to the right person to better, more effectively treat complex disease? This is the promise of precision medicine, to be able to approach complex disease treatment and prevention by taking into account individual variability in genes, environment, and lifestyle for each person.