Ace. Beefsteak. Big Boy. Kumato. Early Girl. Roma. Sun Gold. San Marzano. These are just a few of the thousands of varieties of tomato plants available today. And while all of these varieties may be very different with respect to crop yield, disease resistance,...
Tags
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.

Characterize and Monitor Host Cell Proteins (HCPs) Using SWATH Acquisition Technology
During drug development, the removal of impurities and purification of a final drug product is absolutely essential in order to ensure the safety and efficacy of a therapeutic drug. Of particular concern for biologics are impurities that can stem from host cell proteins. Because biologics are developed through cell culture and fermentation within a host cell, proteins from this host cell can be co-purified with the final biologic. These host cell proteins or HCPs can cause the final product to have undesired side-effects such as eliciting an immune response in patients taking the drug, or affecting the drug’s stability or efficacy. As a result, regulating agencies require drug companies to monitor levels of HCPs during the development and purification of a biologic and to remove HCPs to an acceptable level in the final biotherapeutic product.
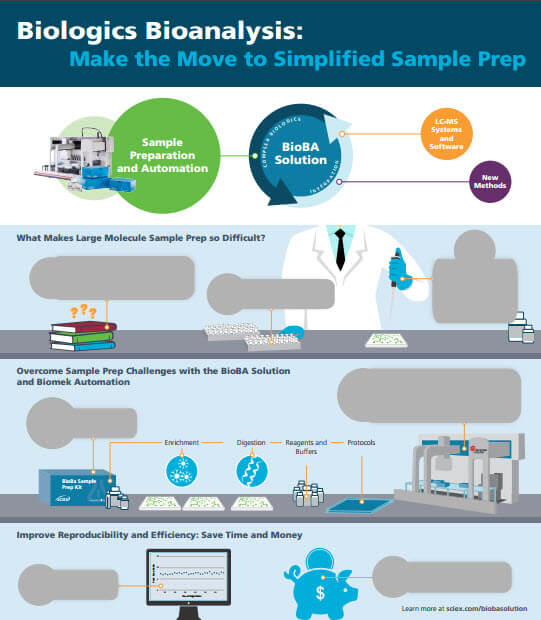
Simplifying Biologics Bioanalysis Sample Prep
These days, everyone seems to be furiously scratching tickets to become instant winners, but I’ll bet you didn’t expect to find sample prep tips that way. For large molecule bioanalysis, preparing your samples can be one of the biggest challenges. It’s a whole different world from traditional small molecule bioanalysis. SCIEX has developed techniques and automation that make biologics sample prep simpler and faster, with reproducible results.
Taking on Precision Medicine with Industrialized Proteomics
What if we could deliver the right treatment at the right time, to the right person to better, more effectively treat complex disease? This is the promise of precision medicine, to be able to approach complex disease treatment and prevention by taking into account individual variability in genes, environment, and lifestyle for each person.

The Connection Between Mass Spectrometry and Space Exploration
Mass spectrometry has been used for some pretty fascinating applications in our world – like testing for steroid use in athletes1, measuring pesticides in grapes2, assessing the efficiency of a psoriasis drug3, and whether that expensive bottle of 100% olive oil is, well, really 100% olive oil.4 But did you know mass spec is also used out of this world? Like… in space?
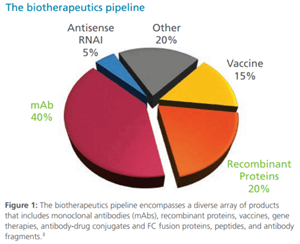
The Future of Biologics Drug Development is Today
Since the 1982 approval of Eli Lilly’s recombinant human insulin, Humulin, biotherapeutic drug development has steadily grown into a global market valued at $140 billion in 2013, increased from $25 billion in 2001
A Hybrid LBA/LC-MS Assay – Your Questions Answered
Last week we posted a blog on Biologics Bioanalysis Key Challenges, where we presented a webinar on those key challenges.
Using Mass Spectrometry to Identify and Quantify Contaminants in Water Samples
Access to clean wholesome water is one of our basic human rights. Human engineering has designed incredible methods to collect, filter, purify, store and distribute water to billions of us worldwide, but does this mean that our water is completely safe to drink? Also,...

LC-MS/MS Method for Biotherapeutic Drug Development Challenges
Traditionally, the pharmacokinetic profile of biotherapeutics such as insulin glargine, adalimumab, trastuzumab and others, used gold standard LBAs to assess dose-response during drug discovery and development. However, LBAs require a specific antibody reagent to be developed for each mAb variant, a process that is often incompatible with the compressed timeframes encountered during the initial stages of drug development.

QTOF Technology for Targeted and Unknown Forensic Drugs Screening Workflows
In this study, the Wisconsin State Laboratory of Hygiene (WSLH) outlines the comparison of their existing technology and how SCIEX LC-MS/MS systems can assist them in their forensic research. The WSLH routinely analyze for 300 forensic drug compounds in over 18,000 samples per year.

Expert Advice to Help You with Routine Food Testing in the Lab
Between 3-6 November 2015, the Recent Advances in Food Analysis (RAFA) 2015 Symposium took place in Prague, Czech Republic.

Contaminants and Novel Approaches in Food Analysis
During RAFA 2015, New Food Magazine hosted a roundtable (sponsored by SCIEX) to bring together experts with routine food testing backgrounds to discuss the latest industry trends, challenges, recent technological advances, and expectations of future laboratories.

Polar Pesticide Analysis by CESI-MS for Routine Food Testing – A Poster Talk
Method development for routine food testing presents many challenges – whether you are looking to increase the speed of your screening or simplify your method there can be different solutions suited to the task at hand. During RAFA 2015 in Prague, Steve Lock, Market Development Manager for SCIEX Separations in EMEA outlines how CESI-MS may be best suited for polar pesticide analysis.

Using Mass Spectrometry to Detect Trace Ingredients in Food – a Poster Talk
In today’s poster talk Jens Dahlmann, Senior Applications Specialist at SCIEX discusses how mass spectrometry might be best suited to the identification of trace ingredients in foods.

How You Can Detect Pesticide 1080 In Milk & Infant Formula – A Poster Talk
In this poster talk André Schreiber, Applications and Product Manager for Food and Environmental Markets at SCIEX guides you through a new method developed in conjunction with Association of Analytical Communities (AOAC). The method is designed to better detect a harmful substance that the infant formula and milk industry are under threat from – Sodium Fluoroacetate, otherwise known as Compound 1080 or Monofluoroacetate.
Drug Metabolism and Bioanalysis Journal Articles Focus on Drug Metabolism and so Much More
Check-out the top articles that focus on innovations in drug metabolism as well as small molecule quantitation and biologics bioanalysis. Exciting advancements! We can’t wait to see all that will come in 2016! Quantitative analysis of maytansinoid (DM1) in human serum...

Reasons why LC-MS/MS is my method of choice for meat speciation and authenticity testing
The meat trade hasn’t had a good reputation since the horsemeat scandal that burst into headlines in 2013. The scandal has left such a mark that meat speciation, adulteration and authenticity are still high-profile topics today. Think about it from a...

Purchasing Mass Spec Technology for Your Forensic Lab
What does every scientist think about in the lab? Validation. This is the feeling I encountered while reading a recent scientific report on nature.com. What struck me was not only the study itself which discounted cannabinoid incorporation into hair as a method for...

A Reliable Method for the Identification, Quantitation, and Confirmation of Pesticides
When carrying out routine pesticide identification tests in your lab how simple is the process of identification, quantitation, and final confirmation from sample to sample? A reliable method designed to generate multiple data sets and confirm sample data in parallel with your test can save an awful lot of time and effort which is especially helpful as the demand for routine testing increases. In this poster talk, Detlev Schleuder, Support Manager for Food & Environmental Markets, explains how the new QTRAP® 6500+ system can optimize your laboratory’s output with this simple method.

The Gluten Free Cookie Label Test
I am a label reader. I like to eat healthily and know what the long, confusing ingredients on the side of a package mean. Therefore, in the spirit of the holiday season, I dedicate this blog to all the gluten intolerant folks out there whose only wish is to eat a yummy cookie while also being absolutely positively sure it is gluten free.

Testing Liquor for Authenticity Using LC-MS/MS Technology
The Proof is in Your Holiday Drink If you think bootlegging was limited to the age of Prohibition then you have never tested liquor for authenticity using mass spectrometry. Maybe it is a scientist thing, but we simply cannot help but bring up the subject as people...