As a researcher in a busy lab, the software driving your work is critical to your success, and the timely transition of SCIEX applications to Windows 10 is no exception. In early 2020 Microsoft will be ending Windows 7 support, and we want you to know we are taking...
Tags
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.
Is Your Lab Prepared for Testing? The Global Supplement Market is Growing
Don’t judge a nutritional supplement by its label, as often, government monitoring of ingredients begins after the product enters the consumer market1. Meanwhile, there may be additional additives not mentioned on the label as they are used to address supplement side effects. Such is the case in the United States where even though federal law requires supplements to carry a dietary supplement label or a substitutional term, monitoring begins once a supplement is on the market. In China meanwhile, the China Food and Drug Administration’s (CFDA) health product potential illegal additives list, clearly stipulates monitoring processes for additives in six different types of nutritional supplements including weight loss, blood sugar reduction, blood pressure reduction, anti-fatigue, sleep improvement and immune strengthening functions.Read Tech Note >

How to Detect Additives in Cosmetics Amongst Ever Changing Regulations
In today’s technical blog, I’m talking about the cosmetics industry so let’s get right to it. According to a Research and Market report, “The Global Cosmetic market was $460 billion USD in 2014 and is estimated to reach 675 billion USD by 2020, growing at a rate of 6.4%.”1 The U.S. leads the pack with a reported $62 billion in revenue earned in 20162. So, what am I getting at? We know earnings are strong and consumers like their products. But the question remains, are these products that you put on your skin, hair, and ingest safe? Such is the thinking of scientists like me and other chemists who are routinely tasked with detecting minimal levels of potentially harmful ingredients in personal care products against ever-changing global regulations.

A Mine of Quantitative Proteomic Information
The Aebersold group at ETH Zurich focuses on proteomics research, including the development of techniques to study the proteome as an integrated entity. In collaboration with SCIEX, the group established SWATH® Acquisition mass spectrometry, a data-independent acquisition (DIA) method capable of fragmenting multiple peptide species concurrently. The resulting comprehensive data set can be retrospectively re-mined, enabling maximum benefit to be derived from any study.

Delivering New Biologics to the Marketplace
Characterization and quantification of host cell proteins (HCPs) in biopharmaceutical development and manufacturing is a critical step to ensuring product safety. While this can be achieved using ELISA, mass spectrometry using the SCIEX TripleTOF® 6600 System is more specific and enables the identification and quantitation of each of the individual proteins present.
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.

The Benefits of Using SWATH Acquisition Technology when Testing Pesticides in Food
Up until recently, SWATH® Independent Data Acquisition (IDA), was not widely used for the detection of pesticides in food samples. Introduced in 2012, SWATH Acquisition is an advanced acquisition technology capable of running on high-resolution mass spectrometers such as the X500R QTOF system or Triple TOF technology. Originally used in the Omics market to ID and quantify complex samples, SWATH Acquisition is gradually making a transition across markets including the investigation of pesticides in food. Like designer drugs, pesticides continuously undergo synthesizing, and food labs are beginning to require a more reliable analysis method to be confident in their resulting reports.

Single Injection, Routine Antibiotic Testing in Urine Samples
The consumption of pharmaceuticals and personal care products is a day to day occurrence. Once consumed the body excretes the remaining part of the compound which is not absorbed. This waste, flushed down the toilet, makes its way through the sewage system before arriving at a treatment facility where it was then processed with chemicals to ensure its cleanliness. Despite being washed, there can remain trace amounts of bacteria, hormones, metals, and antibiotics in whatever you consume, not just water
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.

Food Allergies – How Allergic Are You?
A recent study published by the Annals of Allergy, Asthma, and Immunology (ACAAI), pointed out, in a study of 109 people tested, that skin prick tests are not 100 percent reliable. In the study, participants were subjected to oral food challenges prior to skin testing in which 50 percent of individuals had no reaction. It was also discovered that blood tests were not full-proof even though they measure the presence of IgE antibodies to specific foods. These results are not surprising given that 50 to 60 percent of tests result in false-positives.
Comprehensive Therapeutic Protein Characterization Using One Single Method
Get to know how CESI-MS will allow you to quickly and accurately characterize protein therapeutics for attributes in a single method by downloading this discovery kit.

A Fresh Approach to Food Safety
The EU Reference Laboratory (EURL) for Fruits and Vegetables in Almería is responsible for a network of around 200 laboratories which provide essential surveillance and monitoring to ensure the safety of foodstuffs available across Europe. The EURL provides proficiency testing and method development for these official laboratories, ensuring rigorous screening to avoid harmful chemicals entering the food chain
The Only Solution You Need for Fast MetID
If you work in the breakneck world of therapeutic development, then you probably don’t even have time to read this blog (but we thought we would write it anyways, just in case). Drug metabolism samples are coming into your lab fast and furious. You need to turn them around in hours so that chemists and biologists can optimize the effectiveness of the therapeutic candidate. Time for lunch? We don’t think so!

How Easy Is It to Relocate a Mass Spectrometer?
When you’re in the process of moving your lab, across the corridor or to another country, there’s a lot to think about. Adding to the stress, there’s not always a lot of time to plan, or budget allocated for the process, especially in the case of unexpected urgent maintenance work.

The Key to Measuring Chemical Dyes in Food is LC-MS/MS
Adding colorful dyes to food is nothing new. In the early 19th century, for example, it wasn’t uncommon for manufacturers to add chalk to white bread, thicken milk with a lead compound, and inject red dye into meat in the quest for a fresher appearance1. Fast forward to the 21st century, however, and along with mass spectrometry, food standards have come a long way. Foods now must pass muster according to standards set by government regulators or else risk fines and punishment which can be costly for the manufacturer. To support these measures, are agencies such as the US-FDA, EFSA, and others which have banned some colors due to their toxic and carcinogenic nature which brings me to mass spectrometry analysis. Discover more when you read the following application note, “LC-MS/MS Analysis of Emerging Food Contaminants,” in which researchers used the ExionLC AD with a Phenomenex Column for sample separation followed by MS/MS detection with the SCIEX X500R QTOF system.

What is your Method for Separating Challenging Polar Molecules?
From small ions like phosphate, herbicide degradation to metabolites, oligosaccharides, peptides, and proteins. How is your lab analyzing polar molecules? The reason I ask is there is a saying, if you have a charged or polar molecule, look to capillary electrophoresis (CE) first. While liquid chromatography (LC) is an ideal front-end separation tool for many types of molecules, as the following poster points out, “From Small to Very Large, Orthogonal, Sensitive Polar Molecule Analysis by CESI-MS,” there are some situations that call for CE over LC analysis. For those of you that are not familiar with CESI-MS, it is the combining of CE separation with electrospray ionization, into one dynamic process, within the same device.

The Trouble with PFAS in Drinking Water
There has been a string of news articles concerning polyfluorinated alkyl substances (PFAS) in drinking water these days, and I must say, they have my attention. Here is the thing, when you think of drinking water in the United States, for example, crystal clear lakes, rivers, and groundwater along with effective water treatment come to mind. On the flip side, as safe, some supplies may be there are communities such as that of Flint, Michigan, which have been dealing with lead filled pipes for far too long. Contamination was so bad there that residents were provided bottled water for drinking purposes as the state decided who was responsible for replacing the affected water lines.

SWATH Acquisition – Master of All Trades
SWATH® Acquisition is an innovative strategy for acquiring data on a TripleTOF® mass spectrometer. In a previous blog, we learned how SWATH works. Now let’s learn what it can do for different applications:
The Power Behind LC-MS for Quantifying mAb Therapeutics
Quantitation of monoclonal antibodies (mAbs) in biological fluids is important during all stages of antibody drug development. First developed in the 1970s, therapeutic mAbs have both research and medicinal impact as they can be used for diagnosis and treatment of a wide variety of diseases, and have a high level of specificity.

Data Independent Acquisition Mass Spectrometry with the Power of SWATH
There are many different methods in use today to acquire data on a mass spectrometer, but few have generated as much buzz in recent years as SWATH technology. First reported 5 years ago by Ruedi Aebersold and his group1, SWATH® Acquisition on a TripleTOF® instrument has rapidly become one of the premier acquisition strategies for identification and quantitation of complex samples. But what exactly is SWATH and why is it so powerful? In order to answer these questions, let’s first take a step back and look at the larger picture.
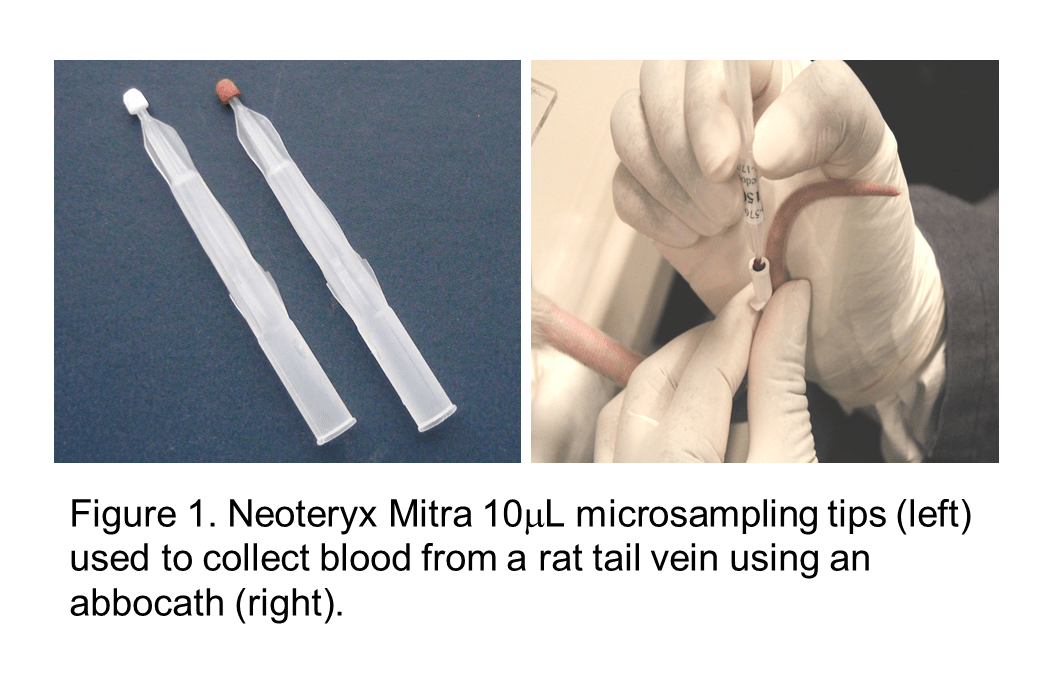
Volumetric Absorptive Microsampling and the SCIEX 6500+: A Pre-Clinical Case Study for the Biotherapeutic Exenatide
In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual