This is the third and final post in our series in honor of International Women’s Day and our collaboration with World Cancer Research Fund. To wrap up, Deborah Howland talks about the importance of diet for anyone facing cancer – or trying to prevent it. A specialist...
Tags

Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

Maximize NPS analysis with accurate mass spectrometry
LC-MS/MS is a powerful analytical tool in forensic toxicology testing that can support a variety of testing regimes such as screening, confirmation and quantitative workflows. More specifically, analysis of NPS using LC-MS/MS provides many advantages, including the ability to reliably detect new drugs and their metabolites from a variety of biological matrices.

Unlock the benefits of nominal mass spectrometry for NPS analysis
The development of analytical methods for the detection and quantitation of drugs and metabolites in a range of biological matrices is a challenging process. Forensic toxicology labs need a reproducible and reliable methodology to ensure the robustness of the data and the quality of the results. They also need robust and sensitive instrumentation that can detect drugs at trace levels with high specificity, especially when it comes to novel psychoactive substances (NPS), which can be difficult to monitor and control.

What has the Echo® MS system done for the pharma industry? (And don’t just take our word for it!)
SCIEX was very proud to have an illustration of the Acoustic Ejection Mass Spectrometry (AEMS) technology that powers the Echo® MS system on the front cover of the Journal of the American Society for Mass Spectrometry in January 2023. The associated article—Ultrahigh-Throughput Intact Protein Analysis with Acoustic Ejection Mass Spectrometry—was co-authored by scientists from SCIEX and Merck.

Back to the new basics: Part 1 | Making the leap from GC-MS to LC-MS
Producing accurate results quickly in a demanding environment is no easy feat for analytical scientists. What’s more, many of us are constantly questioning ourselves—I certainly am—about whether we are employing the best technique for the analysis at hand.
It’s an overwhelming thought, considering the wide range of tools that are available to choose from, each of which offers varying levels of capacity, sensitivity, selectivity, specificity and cost. How do you meet the unique needs of your organization without breaking the bank? I get it, and I’m not here to convince you it’s easy. My aim is to guide you through the process to help you make the right decision for you.
How does automatic calibration work on the QTOF, TripleTOF and ZenoTOF systems?
When you think about calibration of a mass spectrometer, there are actually two aspects to consider. There is the accuracy of the calibration (that it is correct when calibration is performed) and the stability or precision of the mass calibration (that it remains...
What is SWATH acquisition and what are the critical acquisition attributes?
In data-independent acquisition strategies like SWATH acquisition, an expanded mass isolation window is stepped across a mass range covering the mass-to-charge (m/z) distribution of peptides and a full scan MS/MS spectrum is collected at each step. Post-acquisition,...
Controlling the M5 MicroLC system with SCIEX OS software using contact closure
Contact closure can be used to control external devices that are not directly controlled by SCIEX OS software. A sample batch is first created in the SCIEX OS software for MS acquisition, and then a similar batch is created on an external LC device with the required...

The risky business of aflatoxins in milk
If you’re in the dairy or food testing business, you know the threat aflatoxins pose. Aflatoxins are a type of mycotoxin produced by Aspergillus parasiticus, aspergillus flavus , and rarely aspergillus nomius.1 These are likely the most extensively researched group of mycotoxins because of their adverse health effects.2 What’s more, they are widely found in a variety of crops, namely maize, tree nuts, and spices. Believed to be primarily caused by rising temperatures and humidity, these naturally occurring fungi grow on crops in the field, or during storage of feed and raw materials, where they can potentially produce toxins that enter the food chain.
See How Easy It Can Be to Get Expert Results for Biologics Characterization
Learning a new mass spec system can be a daunting task. Aside from the opportunity costs of training new users, you might face the hassle of downtime, and the wait to get expert help when needed. The X500B QTOF system puts a new spin on biologics characterization workflows because it is so easy to learn and operate that you can be up and running much faster than you expect. Powerful new software tools dramatically streamline method development and data processing, to enable everyone in your lab to get expert results. It’s fast because it’s easy, even for new users.

Getting a Clean Match in Forensic Toxicology using LC-MS/MS
As a forensic scientist, what holds you back in the lab? It’s a question we often ask ourselves here at SCIEX, as product development depends on customer wants, needs, satisfaction, and ease of workflow. Ensuring evidence can withstand forensic scrutiny, for example, correlates with the integrity of testing procedures. Knowing this, how do you convince your staff to be confident in results, or convey technical data to a non-technical courtroom audience? If you have been left wondering how to get to the bottom of topics like these, check out the following toxicology toolkit. It’s a bundle of resources at your fingertips that includes a webinar led by Tania A. Saski Ph.D., Northwest Physician Laboratories, Bellevue, Using QTRAP® Technology to Provide Accurate Identification and Confirmation Beyond a Reasonable Doubt, and so much more

Looking to Quantify and Identify Pesticides in your Food Samples?
Visit our offices on any given day, and you are likely to discover researchers putting mass spectrometry to the test. The hum of the mass spectrometer is as common as conversations as scientists are tasked with developing methods that can be applied in real-world lab scenarios. In this case, André Schreiber SCIEX, Concord, Ontario, Canada, detailed, Comprehensive Quantitation and Identification of Pesticides in Food Samples Using the SCIEX UltraLC 100* and the SCIEX QTRAP® 4500 System.

Discover the New X500B QTOF System, the Simpler, Faster Path to Biologics Characterization Answers
Have you ever wished for a compact instrument that delivers expert-level answers to your most complex biotherapeutic characterization challenges faster and easier than what you are doing now? At SCIEX, we recognize that even expert users want easier ways to perform daily characterization tasks and get great results every time. That’s why we set out to develop the X500B QTOF system: a robust and reliable new instrument and software solution that reduces complexity and simplifies biologics characterization workflows so every scientist can get expert-level results
How to Achieve Higher Sensitivity with Hybrid Immunoaffinity LC-MS Assays
Protein-based biotherapeutics, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) are a growing component of pharmaceutical companies’ drug pipelines. The growth of ADCs in particular is due to their ability to selectivity target and deliver a potent molecule to a cancer cell based on a specific tumor marker. In order to support this growing class of new drug molecules, robust and reliable bioanalytical methods are required. While ligand binding assays (LBAs) like ELISA have been the most popular platform for biotherapeutic quantitation, bioanalytical scientists have been increasingly adopting hybrid LBA/LC-MS methods in this area.

The Application of Research Grade MetabolitePilot™ Software for the Determination of the Catabolic Peptide Products of Exenatide
The stability of peptide and protein biotherapeutics directly impacts their pharmacokinetic profile, efficacy, and safety. It is therefore essential to characterize the stability of a given bio-therapeutic including both in-vivo and in-vitro catabolism, thereby...

LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots
There is a lot you can tell from a droplet of blood as it’s snapshot of what could be present in a body at any given moment. In the following application note, LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots, researchers did just...

LC-MS/MS Analysis of Emerging Food Contaminants
Ever wish you had access to the most up to date application methods but don’t know where to find them? The Food and Beverage Compendium is your one-stop resource for research notes ranging from pesticides, allergens, and antibiotics to mycotoxins, vitamins, and...

Is Your Beverage Truly 100% Fruit Juice?
There is nothing like the flavor of fruit juice whether freshly squeezed or made from concentrate to clench your thirst, except when it’s not 100 percent juice after all. As the following tech note, “Authenticity Assessment of Fruit Juices using LC-MS/MS and...
Superbugs, Antibiotic Resistance, and the QTRAP® 6500+ System
‘Superbugs’, or bacteria that have developed antibiotic resistance as a result of adapting to the drugs used in their treatment, are dangerous infections that doctors struggle to stop from spreading. Even common infections such as urinary tract infections and...
Vice President Biden Announces Agreement Naming Children’s Medical Research Institute’s ProCan Lab to the ‘Cancer Moonshot’ Initiative
A key goal of the ‘Cancer Moonshot’ initiative is the advancement of precision medicine, with the goal of making more targeted therapies available to more cancer patients. And researchers believe that the time is right, with the new technological innovations, the new insight into the biology of cancer and big improvements in the handling of ‘big data.’
It’s Time to Enhance Your Food Testing
Are you looking for ways to up the ante on your LC-MS/MS when it comes to food testing? Researchers here have developed a method for the analysis of approximately 400 pesticides in food samples, and their work is available for viewing in this year’s compendium.

Top Five Misconceptions about Mass Spectrometry
Do you work in a lab handling precious samples yet, hesitant to make the move to mass spectrometry? Many laboratories just like yours continue to conduct sample analysis using ELISA assays, PCR scans, and amino acid tests because of their effectiveness. These processes work, so why change? Well, these type of analytical experiments can report false positive and negative results. You have trained your staff, know the process, and fingers crossed, not too many user errors have compromised analysis.

Stoller Biomarker Discovery Centre, Addressing Some of the Biggest Issues in Medicine
The Stoller Biomarker Discovery Center, developed in partnership with SCIEX, was created to develop new omics technologies for biomarker research to understand the root cause of diseases such as cancer, cardiovascular disease, and autoimmune diseases. We initially announced our collaboration with the University of Manchester back in October 2015.

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.
The History of Isotopic Labels for Quantitative Proteomics
Proteomics has become a vital tool for biological scientists performing research on the healthy and diseased states of living things. It involves the large scale and systematic analysis of all proteins within a given cell, tissue, or organism. Because proteins are regulated by many different internal and external stimuli, the proteome is dynamic and quantities of proteins can change from one state to the next. Therefore, in order to be of the highest utility, proteomics experiments need to both identify and quantify proteins so that comparative studies can be done, such as between healthy cells and tumor cells, or the comparison of different treatment regimens.

Struggling to Analyze Small Volume Samples with Conventional LC-MS?
The M3 MicroLC System is designed for scientists who are struggling to analyze small volume samples with conventional LC-MS and need to lower their limits of quantitation while maintaining throughput and robustness. When designing the M3 MicroLC System, we...

Legal and Illicit Drugs in Wastewater Detected and Confirmed with QTRAP Technology
What happens when you up the sensitivity and lower detection limits on influent and effluent sewage tests? For starters, low levels of illegal drugs in samples begin to emerge. This is what researchers discovered when they combined the power of LC-MS/MS with the...
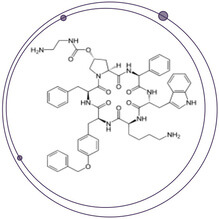
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...