A few months back, the American Academy of Pediatrics published a technical report on the use of chemicals in food processing and the negative health effects on children. One of the main culprits is phthalates. The 411 of PhthalatesPhthalates are esters of phthalic...
Tags

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.
Easy switching of sources and LC flow regimes on the ZenoTOF 7600 system
This series of videos outlines how a user can easily switch sources and operate the system in different LC flow regimes.

Thailand cannabis legalization
Thailand has become the first southeast Asian country to legalize cannabis for medical use. Cannabis was originally introduced into Thailand from India, and until it was outlawed in the 1930s, it was historically used as a kitchen condiment, medicine and source of fiber.
Uploading and using transcriptomics data in the OneOmics suite
RNA experiments can be created in the OneOmics suite for multi-omics analyses, enabling integration of transcriptomics data and proteomics data for biological insight. To build RNA experiments, either CloudConnect for PeakView software 2.2 or BaseSpace (Illumina) can...
Downloading results from SCIEX Data Store or BaseSpace
Once your data processing sessions have completed, the results files are saved back to either SCIEX Data Store or BaseSpace. These can be downloaded from the cloud to your desktop for additional analysis. Please see these community posts to learn more: Explaining the...

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

The top 5 questions to ask when investing in accurate mass technology for forensic toxicology workflows
Are you considering the purchase of a high-resolution accurate mass (HRAM) instrument for your forensic toxicology lab? To help ensure you invest in a solution that ideally meets your needs, ask yourself the following key questions. 1. How do I ensure my results...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...

LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots
There is a lot you can tell from a droplet of blood as it’s snapshot of what could be present in a body at any given moment. In the following application note, LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots, researchers did just...

LC-MS/MS Analysis of Emerging Food Contaminants
Ever wish you had access to the most up to date application methods but don’t know where to find them? The Food and Beverage Compendium is your one-stop resource for research notes ranging from pesticides, allergens, and antibiotics to mycotoxins, vitamins, and...

Is Your Beverage Truly 100% Fruit Juice?
There is nothing like the flavor of fruit juice whether freshly squeezed or made from concentrate to clench your thirst, except when it’s not 100 percent juice after all. As the following tech note, “Authenticity Assessment of Fruit Juices using LC-MS/MS and...
Superbugs, Antibiotic Resistance, and the QTRAP® 6500+ System
‘Superbugs’, or bacteria that have developed antibiotic resistance as a result of adapting to the drugs used in their treatment, are dangerous infections that doctors struggle to stop from spreading. Even common infections such as urinary tract infections and...
Vice President Biden Announces Agreement Naming Children’s Medical Research Institute’s ProCan Lab to the ‘Cancer Moonshot’ Initiative
A key goal of the ‘Cancer Moonshot’ initiative is the advancement of precision medicine, with the goal of making more targeted therapies available to more cancer patients. And researchers believe that the time is right, with the new technological innovations, the new insight into the biology of cancer and big improvements in the handling of ‘big data.’
It’s Time to Enhance Your Food Testing
Are you looking for ways to up the ante on your LC-MS/MS when it comes to food testing? Researchers here have developed a method for the analysis of approximately 400 pesticides in food samples, and their work is available for viewing in this year’s compendium.

Top Five Misconceptions about Mass Spectrometry
Do you work in a lab handling precious samples yet, hesitant to make the move to mass spectrometry? Many laboratories just like yours continue to conduct sample analysis using ELISA assays, PCR scans, and amino acid tests because of their effectiveness. These processes work, so why change? Well, these type of analytical experiments can report false positive and negative results. You have trained your staff, know the process, and fingers crossed, not too many user errors have compromised analysis.

Stoller Biomarker Discovery Centre, Addressing Some of the Biggest Issues in Medicine
The Stoller Biomarker Discovery Center, developed in partnership with SCIEX, was created to develop new omics technologies for biomarker research to understand the root cause of diseases such as cancer, cardiovascular disease, and autoimmune diseases. We initially announced our collaboration with the University of Manchester back in October 2015.

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.
The History of Isotopic Labels for Quantitative Proteomics
Proteomics has become a vital tool for biological scientists performing research on the healthy and diseased states of living things. It involves the large scale and systematic analysis of all proteins within a given cell, tissue, or organism. Because proteins are regulated by many different internal and external stimuli, the proteome is dynamic and quantities of proteins can change from one state to the next. Therefore, in order to be of the highest utility, proteomics experiments need to both identify and quantify proteins so that comparative studies can be done, such as between healthy cells and tumor cells, or the comparison of different treatment regimens.

Struggling to Analyze Small Volume Samples with Conventional LC-MS?
The M3 MicroLC System is designed for scientists who are struggling to analyze small volume samples with conventional LC-MS and need to lower their limits of quantitation while maintaining throughput and robustness. When designing the M3 MicroLC System, we...

Legal and Illicit Drugs in Wastewater Detected and Confirmed with QTRAP Technology
What happens when you up the sensitivity and lower detection limits on influent and effluent sewage tests? For starters, low levels of illegal drugs in samples begin to emerge. This is what researchers discovered when they combined the power of LC-MS/MS with the...
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...
Harnessing the Power of MRM3 for Large Molecule Quantitative Bioanalysis
In a previous blog outlining the advantages of high-resolution accurate mass measurements for protein quantitation using the TripleTOF 6600, it was noted that although the triple-stage quadrupole demonstrated high sensitivity when operated in multiple reaction monitoring mode (MRM), the relatively low-resolution measurement of m/z failed to discriminate Rituximab response from nominally isobaric interferences given the complexity of the proteolytically digested samples (June 28/2016). While the accurate mass filtering capabilities of the TripleTOF 6600 represents one mechanism for achieving increased selectivity over MRM, the triple quadrupole/linear ion trap (LIT) hybrid platform represented by the QTRAP® 4500, 5500, 6500 and 6500+ systems provides an alternative technique by leveraging a third stage of MS, often referred to as MRM3. In this blog, we outline the MRM3 scan function and survey several large molecule applications which utilize the additional stage of fragmentation in the LIT to yield significant improvements in achievable detection limits when compared to MRM.

Taking care of your mass spectrometer—Onsite troubleshooting and maintenance training for today’s lab
Recently, we asked customers to tell us about their biggest challenges so we could customize training programs to meet the needs of today’s growing lab. Without hesitation, most of you said uptime and employee training are your most critical needs. As a result, our...

A Sting in the Tale for Neonicotinoids
Did you know one out of every three mouthfuls of your meal is a product of honeybee pollination—almonds and other tree nuts, berries, fruits, vegetables? To put numbers behind it, honeybee pollination amounts to about $15 billion of U.S....
The detection of acid herbicides and urons by large volume injection
Pre-treatment versus direct injection – that is the question posed in the application note, “The Detection of Acidic Herbicides and Phenyl Ureas by LC-MS/MS with Large Volume Injection and Automated Column Switching.” It’s just one of the dozens of articles you will find within the Environmental Compendium (pages 1 to 4, pesticides) now available for download.
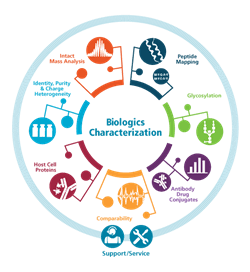
See What More You Can do With 360 Degree Biologics Characterization
Ever wish you had your own team of mass spectrometry experts at your side when working through biologics development and characterization challenges? With SCIEX 360° Innovations complex biologics characterization is streamlined with a full suite of mass spectrometry (MS), capillary electrophoresis (CE) systems, software, and services from SCIEX experts.
The Promise of Precision Medicine
Here is the latest update on the Worldwide Efforts to Accelerate Precision Medicine
The NIH recently issued a press release in early July announcing $55 million in awards. According to the release, the $55 million award in the fiscal year 2016 will go towards building the foundational partnerships and infrastructure needed to launch the Cohort Program of President Obama’s Precision Medicine Initiative (PMI). The PMI Cohort Program is a landmark longitudinal research effort that aims to engage 1 million or more U.S. participants to improve the ability to prevent and treat disease based on individual differences in lifestyle, environment, and genetics.