Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.

Metabolite identification and peace of mind
Managing metabolite identification (Met ID) studies is challenging, so what is at the top of your priority list as you plan the year ahead? Ensuring you have the data needed to manage product safety, meeting deadlines, staff recruitment and training, maintaining compliance, capital expenses, or something else?

What has the Echo® MS system done for the pharma industry? (And don’t just take our word for it!)
SCIEX was very proud to have an illustration of the Acoustic Ejection Mass Spectrometry (AEMS) technology that powers the Echo® MS system on the front cover of the Journal of the American Society for Mass Spectrometry in January 2023. The associated article—Ultrahigh-Throughput Intact Protein Analysis with Acoustic Ejection Mass Spectrometry—was co-authored by scientists from SCIEX and Merck.

New features in OneOmics suite
I just wanted to thank the readers here, both the OneOmics suite users who’ve shared their time and watched OneOmics grow, and for all the talented developers and scientists who’ve made OneOmics suite what it is today.

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

Importing acquisition methods from Analyst software to SCIEX OS software
The SCIEX Triple Quad 7500 system is the first nominal mass instrument to be released completely on SCIEX OS software. Moving to a new software solution can be time consuming with the need to transfer numerous methods to the new platform. SCIEX OS software helps...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

The top 5 questions to ask when investing in accurate mass technology for forensic toxicology workflows
Are you considering the purchase of a high-resolution accurate mass (HRAM) instrument for your forensic toxicology lab? To help ensure you invest in a solution that ideally meets your needs, ask yourself the following key questions. 1. How do I ensure my results...

A rising star in food allergen research: proteomics of shellfish allergen
It’s important to know what you’re eating, especially if you suffer from a food allergy.
About 220 million people worldwide live with a food allergy.1 These numbers, along with the complexity and severity of conditions, continue to rise. In America, there are about 32 million food allergy sufferers—5.6 million of those are children under the age of 18.2.2 That’s 1 out of every 13 children, or about 2 in every classroom. From a financial perspective, the cost of food allergy childcare for US families is up to $25 billion

Calling SCIEX Software Users: Windows 10 Support for all SCIEX Software
As a researcher in a busy lab, the software driving your work is critical to your success, and the timely transition of SCIEX applications to Windows 10 is no exception. In early 2020 Microsoft will be ending Windows 7 support, and we want you to know we are taking...

Detect the Signal, Not the Noise
Improving the specificity and selectivity of your assay Your LC-MS assay is only as good as its power to discern your target compound from everything else. Standards dissolved in clean solvents can make beautiful assays, but analytes in real-world samples can behave...

Software Licensing Comparison: Subscription or Perpetual?
Are you confused by software licensing? Do you want to know the difference between a subscription and a perpetual license? In this blog, we compare both options and explain the value of each choice for your laboratory. Years ago, the perpetual license model was the...
Make the Leap from GC to LC-MS/MS
Choosing the best technique for your analysis can be tough. Should you go with gas chromatography/mass spectrometry (GC-MS) or liquid chromatography/tandem mass spectrometry (LC-MS/MS)? That’s the key question. That’s why we’re here to help. The Limitations of...

Sensitivity, It’s at the Very Heart of Who We Are
Walk into any modern pharmaceutical company these days, and you’ll likely find at least one if not many, SCIEX LC-MS/MS instruments. Assays for the detection and quantitation of small molecule drugs, metabolites, biotherapeutics, biomarkers, and many other analytes...

Uncovering the Links Between Childhood Growth, Body Size, and a Woman’s Risk of Breast Cancer
Welcome to the second in a series of posts marking International Women’s Day, and our ongoing support of World Cancer Research Fund. This installment is a review by Dr. Jennifer Baker, of her work, that, with the help of a WCRF grant, is studying body size and its links to breast cancer. Dr. Baker, Lead Investigator at Denmark’s Frederiksberg Hospital, has a Ph.D. in Human Nutrition from Cornell University. Her research focuses on clinical epidemiology.

A Multi-Omics Database for Tomato Research and Breeding
Ace. Beefsteak. Big Boy. Kumato. Early Girl. Roma. Sun Gold. San Marzano. These are just a few of the thousands of varieties of tomato plants available today. And while all of these varieties may be very different with respect to crop yield, disease resistance,...

Pathway to Success: Young Metabolomics Researchers Named in The Analytical Scientist’s Power List
The direct correlation of the metabolome to the phenotype means metabolomics is one of the most sought-after approaches for the study of disease and wellness, yet the separation, detection, quantification, and unambiguous identification of a chemically diverse...

“Bottoms Up” Proteomics
Ahhhh beer. It's a ubiquitous drink found in over 90% of all countries around the world. Since the dawn of civilization, man has celebrated with beer where it can make even the most introverted person suddenly dance a little jig or belt out a top 40 song. But other...

Metabolomics Studies Benefit Biomedical Research
Professor Dr. Thomas Hankemeier, Head of the Division of Systems Biomedicine and Pharmacology, LACDRLACDR is a center of excellence for multidisciplinary research into drug discovery and development, with a strong focus on metabolomics. As part of its research...
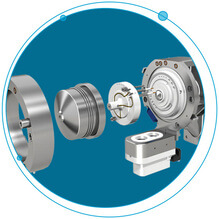
The Ultimate Selectivity for Peptide and Protein Quantitation
There’s no doubt about it, biopharma drug development is experiencing phenomenal growth and presents a variety of challenges not experienced in small molecule development. Some of these challenges are in the selective and sensitive quantitation of peptides and...
Maximize the use of your software. Buy only what you need
The latest releases of Analyst Software 1.7 and SCIEX OS Software 1.4 introduce a new licensing model called concurrent licensing. If you want flexibility and cost savings when purchasing and using your processing software, concurrent licensing is for you. How does...

3 Reasons to Upgrade to Analyst Software 1.7
Would you be surprised to know that the SCIEX QTRAP® and Triple Quad™ mass spectrometry systems are ideally suited to meet the needs of any lab? Even more so as new orders will ship with our flagship Analyst® Software 1.7. The software is the single LC-MS/MS software,...

On Demand Videos from the 2017 Global CESI-MS Symposium
The 2017 Global CESI-MS Symposium brought together KOLs and industry innovators from around the world to share their latest advancements using capillary electrophoresis integrated with electrospray ionization (CESI-MS) within the same device.
Multi-Laboratory Study Highlights the Quantitative Reproducibility of SWATH Acquisition (Nature Communications Paper)
Reproducibility is one of the key tenets of the scientific method. But in a recent survey published in Nature, more than 70% of researchers were not able to reproduce another scientist’s experiments, and more than half could not reproduce their own experiments1. While the reasons for this are many, at least some of them stem from issues inherent in data collection.