Are you considering the purchase of a high-resolution accurate mass (HRAM) instrument for your forensic toxicology lab? To help ensure you invest in a solution that ideally meets your needs, ask yourself the following key questions. 1. How do I ensure my results...
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.
6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.
Ion Formation Control Is the Key to Increasing System Robustness
Traditional mass spectrometry ionization methods such as electronic spray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI), are popular methods for detecting the molecular weight of proteins, peptides, and other biologics. The reason being both...

Why You Need Mass Spec for Veterinary Drug Residue and Antibiotic Analysis
As hospitals struggle with increasingly hard-to-treat antimicrobial resistance (ARM) bacterial infections, related deaths now exceed 700,000 per year globally and are predicted to reach 10 million per year by 2050. There’s no denying that these statistics are both...

Simplify Your Life with a Streamlined Workflow for Multiple Attribute Methodology (MAM)
The effort to fully characterize and release a biotherapeutic to the market can be onerous. Typically, many tests are required to identify and monitor various attributes of the final product in order to ensure the safety and efficacy of the drug. These product quality...

Solving the Mystery of Forensic Quantitative Analysis
Why You Need Differential Mobility Spectrometry for Forensic Mass Spec Analysis Forensics depend on detection of even the smallest compounds to deliver results you can rely on. You need fast analysis methods that provide highly accurate data across a multitude of...

Calling all Bioanalysts: We’re Making Bioanalytical Selectivity Challenges History
High selectivity is a key component of successful quantitative bioanalysis. As a bioanalyst, we need consistently accurate and robust quantitation of small molecule therapeutics and metabolites. Challenged by complicated matrix interferences, high baseline signal, and...
The Latest and Greatest for Improving Lipidomic Analysis by Mass Spectrometry
Differential Mobility Separation (DMS) resolves multiple lipid classes within complex lipid matrices prior to MS analysis to enable more confident identification of lipid species and more accurate quantitation by MS/MS. Lipidomics research has progressed rapidly in...

Out with the “If it Ain’t Broke, Don’t Fix it” Approach. In with Remote Monitoring.
Often by the time you’re aware of a problem, it’s too late, and disaster has already struck. You’re forced to pick up the pieces—downtime, reduced productivity and lost data. When your PC goes down, IT comes to the rescue. That’s a reactive approach. In many cases, IT...

Increase your Lab’s ROI with Remote Monitoring Service for SCIEX LC-MS Systems
Like most organizations, your lab is likely thinking about ways to reduce costs and maximize profits.If so, keep in mind that no matter your area of research, sample volume or locations, remote monitoring services can make a difference in yearly maintenance...
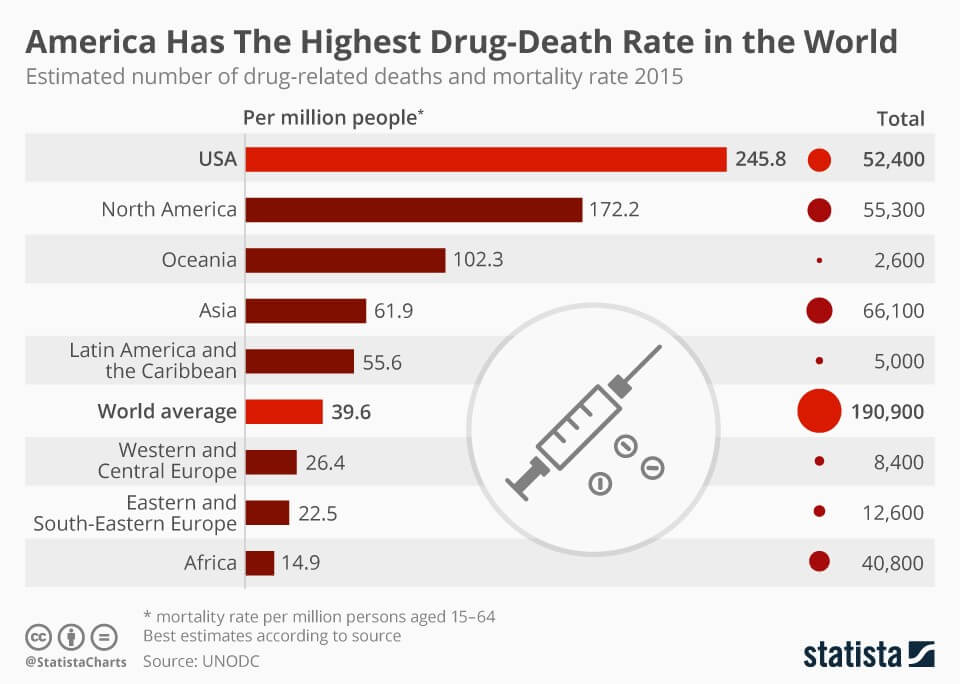
SWATH Acquisition: A Step Closer to Unravelling the Opioid Puzzle
Drug overdose has become one of the leading causes of death of Americans under 50. If that’s not bad enough, let’s put this into perspective. America makes up only 4% of the world population. In 2015, it recorded 52,400 drug-related deaths, which is about 27% of the...

Metabolomics Studies Benefit Biomedical Research
Professor Dr. Thomas Hankemeier, Head of the Division of Systems Biomedicine and Pharmacology, LACDRLACDR is a center of excellence for multidisciplinary research into drug discovery and development, with a strong focus on metabolomics. As part of its research...

Greater uptime equals higher productivity
In the old days, if you wanted to monitor your lab’s data, you would either remain by your instrument as long as it took to complete the sample run or dial-in via a telephone modem. Neither option, however, offered much in the way of enhancing productivity. Today’s...

Simplifying Polar Pesticides Within a Single Analysis
Glyphosate is a polar pesticide widely used as a garden herbicide. It is an ingredient in the world’s bestselling weed killer, which farmers consider one of their best solutions to their super weed problems. However, the chemical has become one of the most...

5 Applications That Benefit from a New Dimension in Selectivity
Mass spectrometry can provide exceptional sensitivity, selectivity, resolution, throughput, and mass accuracy, but certain applications demand additional levels of selectivity. This is where differential ion mobility technology comes in. It delivers highly-selective,...
Maximize the use of your software. Buy only what you need
The latest releases of Analyst Software 1.7 and SCIEX OS Software 1.4 introduce a new licensing model called concurrent licensing. If you want flexibility and cost savings when purchasing and using your processing software, concurrent licensing is for you. How does...

Screening Food for Allergens Using LC-MS/MS Analysis
Browse the shelves of any grocery store, and you may get a false sense of security when it comes to ingredient lists. As much as consumers want to trust labels, the truth is, food products could contain mislabelled ingredients, such that they trigger an allergic reaction with serious detrimental effects including discomfort, pain sickness and in some instances, death. Manufacturers, however, do not want to risk their reputation and consumer safety over a false label. As such, there must be some sort of verification to support such an action

4 Reasons Why Your Lab Needs Remote Monitoring
You know the drill, lab managers are always seeking new solutions to keep their labs running at peak performance and instrument or system disruptions can be detrimental. Wouldn’t it be nice if there were a way to connect to your lab from anywhere securely and to stay ahead of potential instrument problems? By using remote monitoring, labs are now able to respond to issues quickly and efficiently, productively reducing downtime.

3 Reasons to Upgrade to Analyst Software 1.7
Would you be surprised to know that the SCIEX QTRAP® and Triple Quad™ mass spectrometry systems are ideally suited to meet the needs of any lab? Even more so as new orders will ship with our flagship Analyst® Software 1.7. The software is the single LC-MS/MS software,...

Testing for a Variety of Bath Salts is a Necessity for Forensic Labs
To date, when it comes to testing urine or oral fluids in the workplace not all psychoactive substances can be detected due to evolving substitutions. As legislation changes, so too do chemical formulations. Therefore researchers, like the authors of the following publication, A Validated Method for the Detection of 32 Bath Salts in Oral Fluids, published by Oxford Academic, analyze compounds using the best available methods so they can cast a wider net.

On Demand Videos from the 2017 Global CESI-MS Symposium
The 2017 Global CESI-MS Symposium brought together KOLs and industry innovators from around the world to share their latest advancements using capillary electrophoresis integrated with electrospray ionization (CESI-MS) within the same device.
Empowering Your Lab with a Proactive Approach
Do you stay in the lab day and night, waiting for a sample run to finish? Or do you set your instrument to run and trust that it will operate as intended while you are away? Is there a way you can have a support network behind you to help to ensure the smooth operation of your instruments?