We blinked and the last two years went in a flash. It seems like just yesterday, the Dicamba herbicide controversy hit the headlines in 2016 and the EPA set the regulations to expire in two years. Fast forward to today. Dicamba is an acid herbicide used to kill...
Tags

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.
High complexity of the lipidome
The complexity of the lipidome is diverse in the structure and there are many combinatorial isoforms that are available within nature. Currently, many different techniques are required to fully characterize a lipid molecule. What if you could do it in a single...

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...
Full, partial and empty capsid ratios for AAV analysis: What’s the big deal?
For many of you working to develop gene therapy drugs, you know that the time to market the drug is critical. Because gene therapeutics cure diseases by targeting specific genes, it is a constant race to see who develops the drug first. Unlike other classes of drugs where multiple medications can be used to treat a disease, whoever is first to develop a gene therapy drug wins.

Using Mass Spec to Detect Trace Explosives
The importance of protecting a country’s border is a very topical issue. The war on drugs and terror is a 24/7 task 366 days a year (2016 is a leap year). The government agencies in charge must be vigilant and maintain instrumentation to prevent terrorism, drug trafficking, and other illegal activities. Mass Spectrometry is rapidly becoming the instrument of choice for border agencies throughout the world when it comes to explosive trace detection and forensic drug compounds.

Routine Food Testing Using Mass Spectrometry
These days, it is not uncommon to hear about the overzealous application of pesticides to crops or the injection of antibiotics into animals. From grocery stores to restaurants, our food is at risk. How then, can consumers be assured that chemical contaminants like these , not to mention the risk of mycotoxin compounds are not making their way to your dinner table?

Quantify and Identify Pesticides in Complex Food Samples Using the QTRAP 6500 LC-MS/MS System
Recent regulations on food analysis require screening for pesticides using confirmatory techniques, such as GC-MS and LC-MS/MS. More than 1000 pesticides are used worldwide and, along with their metabolites and degradation products, are present in food. There is a demand for powerful and rapid analytical methods that can identify pesticides with high confidence in a broad range of food matrices and quantify at low concentrations with good accuracy and reproducibility. Challenges for pesticide residue laboratories at the moment are the request to test for more compounds, in a wider range of samples, all without sacrificing data quality.

Fast, Efficient, Disulfide Bond Mapping Using BioPharmaView™ Software
Fast LC-MS acquisition and automated data processing will help you speed up peptide mapping of your biotherapeutic, including critical disulfide bond and post-translational modification characterization. SCIEX helps you untangle the complexity of disulfide bonds, speeding up your characterization process.
Bottom-Up Proteomics: A Discussion with Christie Hunter
Biocompare recently featured an article on Bottom-Up Proteomics. I had a chance to follow up with Christie Hunter and expand on some of the questions featured in the article:

Characterize and Monitor Host Cell Proteins (HCPs) Using SWATH Acquisition Technology
During drug development, the removal of impurities and purification of a final drug product is absolutely essential in order to ensure the safety and efficacy of a therapeutic drug. Of particular concern for biologics are impurities that can stem from host cell proteins. Because biologics are developed through cell culture and fermentation within a host cell, proteins from this host cell can be co-purified with the final biologic. These host cell proteins or HCPs can cause the final product to have undesired side-effects such as eliciting an immune response in patients taking the drug, or affecting the drug’s stability or efficacy. As a result, regulating agencies require drug companies to monitor levels of HCPs during the development and purification of a biologic and to remove HCPs to an acceptable level in the final biotherapeutic product.
Simplifying Biologics Bioanalysis Sample Prep
These days, everyone seems to be furiously scratching tickets to become instant winners, but I’ll bet you didn’t expect to find sample prep tips that way. For large molecule bioanalysis, preparing your samples can be one of the biggest challenges. It’s a whole different world from traditional small molecule bioanalysis. SCIEX has developed techniques and automation that make biologics sample prep simpler and faster, with reproducible results.

The Connection Between Mass Spectrometry and Space Exploration
Mass spectrometry has been used for some pretty fascinating applications in our world – like testing for steroid use in athletes1, measuring pesticides in grapes2, assessing the efficiency of a psoriasis drug3, and whether that expensive bottle of 100% olive oil is, well, really 100% olive oil.4 But did you know mass spec is also used out of this world? Like… in space?
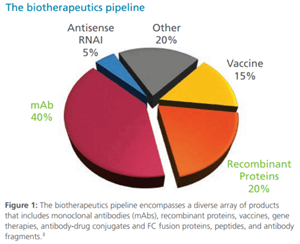
The Future of Biologics Drug Development is Today
Since the 1982 approval of Eli Lilly’s recombinant human insulin, Humulin, biotherapeutic drug development has steadily grown into a global market valued at $140 billion in 2013, increased from $25 billion in 2001
A Hybrid LBA/LC-MS Assay – Your Questions Answered
Last week we posted a blog on Biologics Bioanalysis Key Challenges, where we presented a webinar on those key challenges.
Using Mass Spectrometry to Identify and Quantify Contaminants in Water Samples
Access to clean wholesome water is one of our basic human rights. Human engineering has designed incredible methods to collect, filter, purify, store and distribute water to billions of us worldwide, but does this mean that our water is completely safe to drink? Also,...

LC-MS/MS Method for Biotherapeutic Drug Development Challenges
Traditionally, the pharmacokinetic profile of biotherapeutics such as insulin glargine, adalimumab, trastuzumab and others, used gold standard LBAs to assess dose-response during drug discovery and development. However, LBAs require a specific antibody reagent to be developed for each mAb variant, a process that is often incompatible with the compressed timeframes encountered during the initial stages of drug development.

QTOF Technology for Targeted and Unknown Forensic Drugs Screening Workflows
In this study, the Wisconsin State Laboratory of Hygiene (WSLH) outlines the comparison of their existing technology and how SCIEX LC-MS/MS systems can assist them in their forensic research. The WSLH routinely analyze for 300 forensic drug compounds in over 18,000 samples per year.

Expert Advice to Help You with Routine Food Testing in the Lab
Between 3-6 November 2015, the Recent Advances in Food Analysis (RAFA) 2015 Symposium took place in Prague, Czech Republic.

Contaminants and Novel Approaches in Food Analysis
During RAFA 2015, New Food Magazine hosted a roundtable (sponsored by SCIEX) to bring together experts with routine food testing backgrounds to discuss the latest industry trends, challenges, recent technological advances, and expectations of future laboratories.

Polar Pesticide Analysis by CESI-MS for Routine Food Testing – A Poster Talk
Method development for routine food testing presents many challenges – whether you are looking to increase the speed of your screening or simplify your method there can be different solutions suited to the task at hand. During RAFA 2015 in Prague, Steve Lock, Market Development Manager for SCIEX Separations in EMEA outlines how CESI-MS may be best suited for polar pesticide analysis.

Using Mass Spectrometry to Detect Trace Ingredients in Food – a Poster Talk
In today’s poster talk Jens Dahlmann, Senior Applications Specialist at SCIEX discusses how mass spectrometry might be best suited to the identification of trace ingredients in foods.

How You Can Detect Pesticide 1080 In Milk & Infant Formula – A Poster Talk
In this poster talk André Schreiber, Applications and Product Manager for Food and Environmental Markets at SCIEX guides you through a new method developed in conjunction with Association of Analytical Communities (AOAC). The method is designed to better detect a harmful substance that the infant formula and milk industry are under threat from – Sodium Fluoroacetate, otherwise known as Compound 1080 or Monofluoroacetate.
Drug Metabolism and Bioanalysis Journal Articles Focus on Drug Metabolism and so Much More
Check-out the top articles that focus on innovations in drug metabolism as well as small molecule quantitation and biologics bioanalysis. Exciting advancements! We can’t wait to see all that will come in 2016! Quantitative analysis of maytansinoid (DM1) in human serum...

Reasons why LC-MS/MS is my method of choice for meat speciation and authenticity testing
The meat trade hasn’t had a good reputation since the horsemeat scandal that burst into headlines in 2013. The scandal has left such a mark that meat speciation, adulteration and authenticity are still high-profile topics today. Think about it from a...