A few months back, the American Academy of Pediatrics published a technical report on the use of chemicals in food processing and the negative health effects on children. One of the main culprits is phthalates. The 411 of PhthalatesPhthalates are esters of phthalic...
Tags

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

Detecting low levels of drugs and their metabolites in hair and nail samples using LC-MS/MS
You probably have heard of testing blood and urine samples for the presence of drugs and their metabolites. But do you know about the benefits of hair and nail analysis? In a recent webinar, Tina Binz, Deputy Head of the Center for Forensic Hair Analysis, University of Zurich, discussed the benefits of developing comprehensive and sensitive LC-MS/MS for the detection of low-level drugs and metabolites in keratinized matrices.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
Questions and answers to help improve your mycotoxin analysis
During a recent webinar I shared method details for mycotoxin analysis on the SCIEX 7500 system. In this blog i will share the Q&A for the submitted questions that we did not have chance to answer during the live webinar.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.

Maximize NPS analysis with accurate mass spectrometry
LC-MS/MS is a powerful analytical tool in forensic toxicology testing that can support a variety of testing regimes such as screening, confirmation and quantitative workflows. More specifically, analysis of NPS using LC-MS/MS provides many advantages, including the ability to reliably detect new drugs and their metabolites from a variety of biological matrices.

Metabolite identification and peace of mind
Managing metabolite identification (Met ID) studies is challenging, so what is at the top of your priority list as you plan the year ahead? Ensuring you have the data needed to manage product safety, meeting deadlines, staff recruitment and training, maintaining compliance, capital expenses, or something else?

What has the Echo® MS system done for the pharma industry? (And don’t just take our word for it!)
SCIEX was very proud to have an illustration of the Acoustic Ejection Mass Spectrometry (AEMS) technology that powers the Echo® MS system on the front cover of the Journal of the American Society for Mass Spectrometry in January 2023. The associated article—Ultrahigh-Throughput Intact Protein Analysis with Acoustic Ejection Mass Spectrometry—was co-authored by scientists from SCIEX and Merck.
Methods for OPI electrode cleaning for Echo® MS system electrodes
Depending on the samples you are running on the system, it is possible for the Open Port Interface (OPI) electrode to become dirty or occluded over time. Below are two different cleaning strategies that can help you maintain your Echo® MS system and keep your OPI...

Rescheduling a Schedule I substance, and the Delta-8 controversy
Did you know that in the US, drugs and other chemicals are classified into 5 distinct categories depending on the drug’s acceptable medical use and its potential for abuse or dependency? Drugs federally classified as Schedule I substances by the US Drug Enforcement Administration (DEA) are considered to have the highest potential for abuse and for creating severe psychological and/or physical dependence. In addition to heroin, LSD and MDMA (ecstasy), cannabis is classified as a Schedule I substance in the Controlled Substance Act of 1970, which means it has no approved medical usage.

You’ve Seen It… Now Try It! BioPharmaView Software 2.0
At ASMS this year, the newest version of BioPharmaView Software was released. This software simplifies the processing of biotherapeutic data for characterization and comparability which can dramatically improve your productivity. BioPharmaView 2.0 Software accelerates characterization and comparability studies and simplifies reporting, so you can make better decisions, faster.

Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS
Quantitating antibiotics and insecticides in poultry is serious business. Overuse can lead to antibiotic resistance while insecticide residuals can cause harmful side effects in humans. In the United States, for example, the Federal Drug Administration (FDA), has offered up a plan to limit common antibiotics in feed, which are used to encourage growth. However, this is a voluntary plan, and as the following application note, “Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS,” points out, antibiotics have been shown to accumulate in poultry feathers, which are in turn used for nutritional elements in the feed.

Screening Novel Psychoactive Substances with Confidence
How do you know what you can’t see? This is the challenge many a lab faces as they relentlessly test for novel psychoactive substances (NPS) as unknown samples with an ever-changing ingredient list make discovery difficult work at best. There are many reasons for the complexities of which you can discover in this application note, “Accurate Mass Screening Workflows for the Analysis of Novel Psychoactive Substances.” However, the biggest of which is that non-targeted findings can turn up thousands of molecular features in a single sample. Sifting through the peaks is laborious, and many are normal besides.
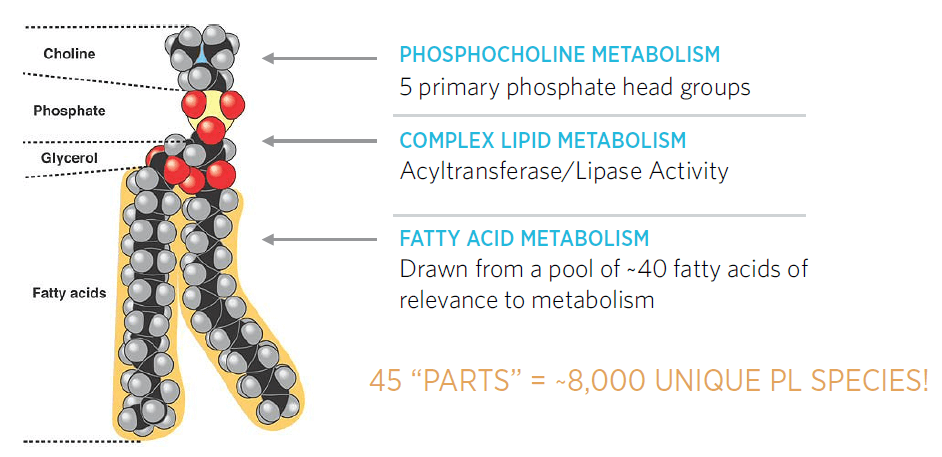
Why Study Lipids?
I had an opportunity to follow up with Steven M Watkins, Ph.D. to talk about the importance of studying lipids in disease. Steve has been working in the lipids field for over 20 years and is one of the foremost experts in lipid biology. Steve founded Lipomics in 2000, an early metabolomics company focused on quantitative lipidomics and had followed that company through a series of changes that led to its involvement in the clinical diagnostic development and global metabolomics. Steve authored over 70 peer-reviewed publications including several book chapters on lipids and lipid metabolism. His presentations on this topic are fascinating and very informative, so I wanted to capture some of his thinking here!

Improved complex sample processing for higher quality of results, reproducibility and depth of proteomic analysis
SCIEX partners to improve depth of proteome coverage
SCIEX and Pressure BioSciences address a major challenge for researchers performing complex sample preparation by marketing a complete solution to increase the depth, breadth, and reproducibility of protein extraction, digestion, and quantitation in all tissue types, especially challenging samples like tumors.

Perfluoroalkyl Acids in Drinking Water – EPA Method 537
The United States Environmental Protection Agency (EPA), under the 1996 Safe Drinking Water Act (SDWA), requires a new list of no more than 30 unregulated contaminants to be monitored by public drinking water systems. Known as the Unregulated Contaminant Monitoring Rule (UCMR), a new list is published every five years. The last rule, UCMR3, was published May 2, 2012, and is the focus of the following application note, “Analysis of Perfluoroalkyl (PFFA) Acids Specified under the UCMR3 Using the QTRAP® 6500 LC-MS/MS system,” which can be found in the Food and Environmental Compendium.

SCIEX helps set food standards in China
One of the biggest concerns of Chinese citizens is food safety1. Even though China ranks second in global economies2, crowding, industrial pollution, labor and certain agriculture practices have contributed to this. In October 2015, however, we began to see a turnaround as the Chinese government revised its 2009 Food Safety Law in an attempt to strengthen its food supply oversight and quality.

Discover the new and accurate SCIEX way to enhance your routine food allergen testing
Food allergy is an immune-mediated, adverse reaction to an antigenic protein. Even limited exposure to an antigen can provoke a significant reaction in sensitive individuals, causing rashes, itching and swelling in the mouth, nausea, vomiting, and asthma. Additionally, food allergies are the leading cause of anaphylaxis, an acute, potentially deadly allergic reaction. The prevalence and severity of food allergies are rising, with approximately 150 million people suffering from food allergies worldwide.1, 2 Presently, there is no cure for food allergies, and sufferers must rely on the correct labeling of foods to avoid consuming allergens. Hence, the development of sensitive and accurate analytical methods to screen for the presence of allergens in food products is necessary for the prevention of potentially life-threatening health problems for allergy sufferers.

Three Infographics to Show You How to Overcome Challenges in Transitioning to Biologics Bioanalysis
The move to large molecules in Pharma is accelerating, offering unprecedented opportunities to improve human health and expand into new markets. But for those with extensive experience with small molecule bioanalysis, the shift to biologics can be challenging, from Sample Prep to Instrumentation and Software, to Methods and Training:

Rapid Characterization of Biologics using CESI-MS
Today, 30 monoclonal antibodies (mAbs), have been approved for the treatment of certain cancers, autoimmune and infectious diseases. Even more are in development, and perhaps you and your team of scientists are working on one now. Keeping pace with fast development timelines while performing comprehensive characterization of biologic candidates can be challenging. However, more and more, scientists are tackling these challenges with new techniques to speed and simplify their characterization workflows. Read more in the application note, “Rapid Characterization of Biologics using a CESI 8000 – SCIEX TripleTOF® System,” found in the Biologics Analytical Characterization Compendium, which highlights how CESI separation coupled with high-resolution mass spectrometry can provide a comprehensive characterization of biotherapeutics.

The Not So Hidden Truth about Climate Change How It’s Poisoning Your Food
Did you know climate change could be poisoning your food? According to the United Nations Environmental Program (UNEP) report on Emerging Issues of Environmental Concern, rising temperatures are making crops more toxic.

Guardians of Antibiotics
This second is a blog series on the global war: Rise of Superbugs! Part 1 took a critical look at the antibiotic threat we face in today’s battlefield. The waning effectiveness of antibiotics as we head into what may seem like a post-antibiotic era has impelled new reformation to at the very least control antibiotic usage to ensure food safety.
Protein Quantitation Workflows using the TripleTOF 6600: A Case Study for Rituximab
Although the triple-stage quadrupole (QQQ) mass spectrometer remains the pillar for quantitative LC-MS/MS bioanalytical assays, due in part to the platforms’ high duty cycle when operated in multiple-reaction monitoring (MRM) mode, the applicability of high-resolution mass spectrometry (HRMS) has become of increasing importance for protein quantitation given the complexity of proteolytically digested samples in the surrogate peptide approach. While the QQQ demonstrates high sensitivity and specificity, the relatively low-resolution measurement of m/z may fail to differentiate analyte response from nominally isobaric background interference. In contrast, HRMS with accurate mass assignment of product ion allows interference to be resolved through judicious selection of a post-acquisition mass extraction window whose tolerance is largely dictated by the effective resolution and stability of mass calibration.

Rise of the Super Bugs
The term “antibiotic-free” is becoming more and more popular in food advertising these days. Take Subway for example; in March the company elevated their antibiotic-free policy and introduced a new antibiotic-free rotisserie-style chicken sub, and they plan to, “Nix antibiotics in all its meat by 2025.”

Glyphosate, a Polar Pest Put to Test
No other pesticide has courted more media attention and controversy in recent months than glyphosate, with governments and national agencies debating its use and health effects.

Using Mass Spec to Detect Trace Explosives
The importance of protecting a country’s border is a very topical issue. The war on drugs and terror is a 24/7 task 366 days a year (2016 is a leap year). The government agencies in charge must be vigilant and maintain instrumentation to prevent terrorism, drug trafficking, and other illegal activities. Mass Spectrometry is rapidly becoming the instrument of choice for border agencies throughout the world when it comes to explosive trace detection and forensic drug compounds.

Routine Food Testing Using Mass Spectrometry
These days, it is not uncommon to hear about the overzealous application of pesticides to crops or the injection of antibiotics into animals. From grocery stores to restaurants, our food is at risk. How then, can consumers be assured that chemical contaminants like these , not to mention the risk of mycotoxin compounds are not making their way to your dinner table?
Industrialize Your Quantitative Proteomics Using a More Simplified Sample Prep
in part 1 and part 2 of this blog series we discussed how you can increase your efficiency for high throughput quantitative proteomics by industrializing your sample analysis and data processing. Microflow SWATH® Acquisition on your TripleTOF® system coupled with OneOmics™ data analysis tools allow you to run samples faster, collect data faster, and process your data files faster. It all adds up to getting more meaningful biological information in a shorter amount of time.

Quantify and Identify Pesticides in Complex Food Samples Using the QTRAP 6500 LC-MS/MS System
Recent regulations on food analysis require screening for pesticides using confirmatory techniques, such as GC-MS and LC-MS/MS. More than 1000 pesticides are used worldwide and, along with their metabolites and degradation products, are present in food. There is a demand for powerful and rapid analytical methods that can identify pesticides with high confidence in a broad range of food matrices and quantify at low concentrations with good accuracy and reproducibility. Challenges for pesticide residue laboratories at the moment are the request to test for more compounds, in a wider range of samples, all without sacrificing data quality.

Industrialize Your Quantitative Proteomics with the OneOmics Project
For many labs, the days are long gone when it was acceptable to run only a few samples a week for your quantitative proteomics projects. The pressure for faster turn-around times, to support larger cohort studies, to sustain multiple research directions, and to transition from a purely unbiased discovery mode to verifying something truly unique and interesting, all demand a faster pace. Many labs are now being asked to analyze a hundred samples a week or more. In part 1 of this blog series, we saw how moving to a microflow SWATH workflow can dramatically increase your throughput with little compromise on overall results. In this part, we’ll address what to do with all of this data because it’s just no good if all we’ve done is move the bottleneck downstream.