It’s been a momentous year for the cannabis industry in Canada. Not only did the country legalize cannabis with the Canadian cannabis law in October 2018, but it has also taken a giant leap in setting up a multi-billion dollar market from scratch. The progress made...
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.
6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
5 Ways That SelexION Technology Will Address Your Biggest Analytical Challenges
If you are a scientist working with complex assays, finding a way to significantly improve selectivity of detection could solve some of your biggest analytical headaches. Are we right? If so, then you are in the right place. If you are confronted with assay...
Multi-Laboratory Study Highlights the Quantitative Reproducibility of SWATH Acquisition (Nature Communications Paper)
Reproducibility is one of the key tenets of the scientific method. But in a recent survey published in Nature, more than 70% of researchers were not able to reproduce another scientist’s experiments, and more than half could not reproduce their own experiments1. While the reasons for this are many, at least some of them stem from issues inherent in data collection.
Why is Metabolomics Important and How is it Enabling Precision Medicine?
Why is metabolomics important?
Metabolomics is the large-scale study of metabolites in biofluids, tissue extracts, or organisms. Metabolites are small (<1000 Da), biologically active molecules such as glucose, cholesterol, creatinine, hormones, lipids, and more.

Training Program for Today’s Food and Beverage Testing Lab
Is your lab looking to acquire methods for food testing? What about getting better acquainted on the SCIEX Triple Quad™ or QTRAP® mass spectrometers to learn quantitation better? The following SCIEXUniversity Success Program training courses not only cover food and beverage quantitation but offer application training on topics such as meat speciation testing and pesticide analysis. Especially important considering the latest Fipronil contamination in eggs.I want to sign up for courses >

Phthalates Are Out, Accurate Detection Using LC-MS/MS Technology Is In
Whether you like it or not, the plastics industry is a growing market. According to an Allied Market Research report, it was valued at $15,179 million in 2015 and is projected to reach $18,538 million by 2022, growing at a CAGR of 2.9% from 2016 to 20221. Additionally, according to the report, in 2015, phthalates type held two-thirds of the global market in 2015. An important statistic since the use of various phthalates is restricted in many countries because of health concerns2.

SCIEX Lands HUPO Science and Technology Award
We are pleased to congratulate its research scientists Stephen Tate and Ron Bonner (retired) for being awarded this year’s Science and Technology award at HUPO 2017 in Dublin Ireland. The Science and Technology Award at HUPO recognizes an individual or team who were key in the commercialization of a technology, product, or procedure that advances proteomics research

Forensics Made Easy
Mass spectrometry techniques are now commonplace for high throughput quantitation and screening, but also for research and discovery for food safety, forensics, environmental testing, and a host of other applications. The demands of these settings are different from traditional research, requiring MS systems which combine robust and reliable operation with straightforward day-to-day processing. The SCIEX X500R QTOF System has been developed specifically to meet these needs and is now helping to streamline the workflow of Zurich’s Institute of Forensic Medicine.
3 Workflows Designed to Accelerate Your Biologics Characterization
Biopharmaceutical development is booming and now an integral part of many pharmaceutical company pipelines. While these emerging biologics present exciting opportunities for the industry, their sophistication is challenging the limits of characterization at all stages of discovery and development.
Making Your Vitamin D Testing Dreams Come True
If you work in clinical diagnostics, you can probably confirm that most clinical laboratories have seen a 5 to 6-fold increase in 25-hydroxyvitamin D testing over the past decade, and volume is growing. Furthermore, the Office of Inspector General (OIG) recently reported Vitamin D as one of the top five laboratory assays reimbursed by Medicare, accounting for 8.7 million laboratory tests and $337 million in reimbursement dollars.
The Science Behind SelexION Differential Mobility Spectrometry Technology
Scientists and analysts across all fields of testing and research are increasingly challenged by complex samples requiring advanced analytical selectivity. And where LC-MS/MS sensitivity alone is not enough to meet the demands of modern day quantitative performance, Differential Ion Mobility Spectrometry (DMS) has proven to be a valuable addition.
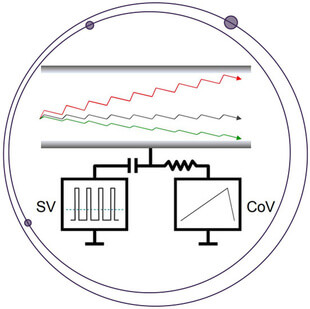
Elimination of Interference using the SelexION Differential Mobility System for the Quantitation of Rituximab in a Dual Surrogate Peptide Approach
The quantitation of proteins using the surrogate peptide approach can complicate nominal mass Triple Quadrupole MRM measurements due to co-extracted interference when using non-selective extraction techniques such as pellet digestion. High resolution coupled with accurate mass filtering can mitigate such interference, as reported previously for the determination of rituximab using the TripleTOF 6600 (Protein Quant Approaches). However, an additional level of selectivity can often be achieved on nominal mass systems using the orthogonal gas-phase separation approach offered by the SelexION+® Differential Mobility System technology (DMS). Interfaced between the sampling orifice and ion source, the DMS separates ions based upon differences in their migration rates under alternating low and high field waveform amplitudes (Figure 1). Ion clustering in low fields and declustering in high fields amplifies the distinction in mobility of an ion, resulting in improved resolution from interfering species of differing molecular cross-section.1-4

Happy Birthday to SWATH Acquisition! 5 Years of Innovation
With its introduction at the HUPO World Congress in 2010 in Sydney Australia by Ruedi Aebersold, SWATH® Acquisition instantly intrigued scientists around the world. Here was a new technique with the potential to revolutionize the way proteomics studies were performed! Based on a data independent acquisition strategy using a SCIEX TripleTOF® 5600 system, SWATH was able to consistently identify and quantify at least as many peptides and proteins as other far more mature proteomics strategies on the market, but with quantitative accuracy and reproducibility rivaling gold standard MRM experiments! This solution was made broadly available to researchers with a full launch of SWATH Acquisition in the Analyst® TF 1.6 Software on the TripleTOF 5600+ System at ASMS 2012 in Vancouver (A Mine of Quantitative Proteomic Information. Prof Dr. Ruedi Aebersold, Head of the Department of Biology, ETH Zurich).

5 Tips for Calibrating a QTOF Mass Spectrometer
Do you have questions about your mass spec? How about a workflow? Our community members are involved in active discussions and receive expert answers from customers like you, SCIEX scientists, and support specialists every week. One recent topic concerned the automatic calibration on TripleTOF® systems as answered by Dr. Christie Hunter whose focus is developing and testing innovative MS workflows for omics research through working collaboratively with the instrument, chemistry, and software research groups.

Enhancing In Vitro ADME Screening
LC-MS technology is helping contract research organization Cyprotex Discovery Ltd. perform bioanalysis of small molecules, peptides, and other pharmaceuticals, enabling quantification to be performed in complex matrices during in vitro ADME studies.
Host Cell Protein Analysis – Mass Spec’s Edge Over ELISA
The number of protein based drugs coming onto the market is at an all-time high, particularly those produced with a host cell system. With host cells come their own proteins. These host cell proteins (HCPs) constitute a major part of process-related impurities and can adversely affect drug safety, so it is critical that they are identified and quantified accurately.
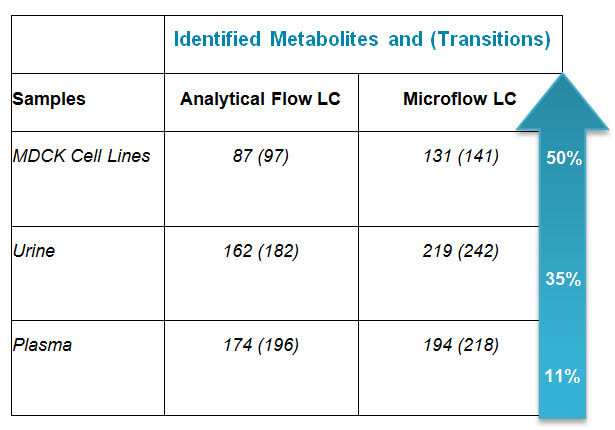
Why Microflow HILIC Chromatography for Targeted Metabolomics Applications?
I recently had the opportunity to catch up with Baljit Ubhi to discuss the top questions you’re asking in regards to using Microflow HILIIC Chromatography for Targeted Metabolomics. Here’s what Bal said:

Fipronil Tainted Eggs Detected in Several European Countries
News agencies all over the world are reporting a new food contamination issue regarding eggs which have been found to contain residues of Fipronil. According to Nieuwsuur, a Dutch news, and current affairs program, “The Fipronil scandal is a huge blow to the poultry sector. Millions of eggs are destroyed and 138 companies remain tentatively closed. But supermarkets also face great damage. In recent days all contaminated eggs have been taken out of the shelves.” CBS news has reported that contaminated eggs have been discovered in Belgium and in the Netherlands with other European countries now on alert.

In Search of the Unknown
The production of high-quality drinking water entails rigorous treatment and testing procedures. For water suppliers’ laboratories, such as the Zweckverband Landeswasserversorgung in Germany, one of the major challenges is the identification of trace levels of organic substances, which can be achieved with the help of mass spectrometry.

Discover The Benefits of Knowledge Base Articles
Did you know you can access Knowledge Base Articles for trending user questions compiled and answered by SCIEX support experts? Doing so may help to reduce your support calls, not to mention downtime. Instead of waiting for a problem to occur, you can stay on top of it, and be a part of the solution. To give you an idea of trending articles, consider the how this past month saw questions and answers including:

A Fleet of Analyzers Keeps Work Flowing
An Interview with Timothy Sangster, Head of Bioanalysis and Immunology, Charles River Laboratories, Edinburgh