Choosing the best technique for your analysis can be tough. Should you go with gas chromatography/mass spectrometry (GC-MS) or liquid chromatography/tandem mass spectrometry (LC-MS/MS)? That’s the key question. That’s why we’re here to help. The Limitations of...
Tags

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.
Easy switching of sources and LC flow regimes on the ZenoTOF 7600 system
This series of videos outlines how a user can easily switch sources and operate the system in different LC flow regimes.

Thailand cannabis legalization
Thailand has become the first southeast Asian country to legalize cannabis for medical use. Cannabis was originally introduced into Thailand from India, and until it was outlawed in the 1930s, it was historically used as a kitchen condiment, medicine and source of fiber.
Uploading and using transcriptomics data in the OneOmics suite
RNA experiments can be created in the OneOmics suite for multi-omics analyses, enabling integration of transcriptomics data and proteomics data for biological insight. To build RNA experiments, either CloudConnect for PeakView software 2.2 or BaseSpace (Illumina) can...
Downloading results from SCIEX Data Store or BaseSpace
Once your data processing sessions have completed, the results files are saved back to either SCIEX Data Store or BaseSpace. These can be downloaded from the cloud to your desktop for additional analysis. Please see these community posts to learn more: Explaining the...

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

The top 5 questions to ask when investing in accurate mass technology for forensic toxicology workflows
Are you considering the purchase of a high-resolution accurate mass (HRAM) instrument for your forensic toxicology lab? To help ensure you invest in a solution that ideally meets your needs, ask yourself the following key questions. 1. How do I ensure my results...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
3 Workflows Designed to Accelerate Your Biologics Characterization
Biopharmaceutical development is booming and now an integral part of many pharmaceutical company pipelines. While these emerging biologics present exciting opportunities for the industry, their sophistication is challenging the limits of characterization at all stages of discovery and development.
Making Your Vitamin D Testing Dreams Come True
If you work in clinical diagnostics, you can probably confirm that most clinical laboratories have seen a 5 to 6-fold increase in 25-hydroxyvitamin D testing over the past decade, and volume is growing. Furthermore, the Office of Inspector General (OIG) recently reported Vitamin D as one of the top five laboratory assays reimbursed by Medicare, accounting for 8.7 million laboratory tests and $337 million in reimbursement dollars.
The Science Behind SelexION Differential Mobility Spectrometry Technology
Scientists and analysts across all fields of testing and research are increasingly challenged by complex samples requiring advanced analytical selectivity. And where LC-MS/MS sensitivity alone is not enough to meet the demands of modern day quantitative performance, Differential Ion Mobility Spectrometry (DMS) has proven to be a valuable addition.
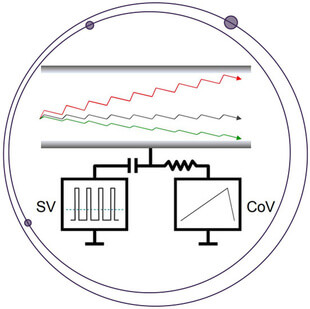
Elimination of Interference using the SelexION Differential Mobility System for the Quantitation of Rituximab in a Dual Surrogate Peptide Approach
The quantitation of proteins using the surrogate peptide approach can complicate nominal mass Triple Quadrupole MRM measurements due to co-extracted interference when using non-selective extraction techniques such as pellet digestion. High resolution coupled with accurate mass filtering can mitigate such interference, as reported previously for the determination of rituximab using the TripleTOF 6600 (Protein Quant Approaches). However, an additional level of selectivity can often be achieved on nominal mass systems using the orthogonal gas-phase separation approach offered by the SelexION+® Differential Mobility System technology (DMS). Interfaced between the sampling orifice and ion source, the DMS separates ions based upon differences in their migration rates under alternating low and high field waveform amplitudes (Figure 1). Ion clustering in low fields and declustering in high fields amplifies the distinction in mobility of an ion, resulting in improved resolution from interfering species of differing molecular cross-section.1-4

Enhancing In Vitro ADME Screening
LC-MS technology is helping contract research organization Cyprotex Discovery Ltd. perform bioanalysis of small molecules, peptides, and other pharmaceuticals, enabling quantification to be performed in complex matrices during in vitro ADME studies.
Host Cell Protein Analysis – Mass Spec’s Edge Over ELISA
The number of protein based drugs coming onto the market is at an all-time high, particularly those produced with a host cell system. With host cells come their own proteins. These host cell proteins (HCPs) constitute a major part of process-related impurities and can adversely affect drug safety, so it is critical that they are identified and quantified accurately.
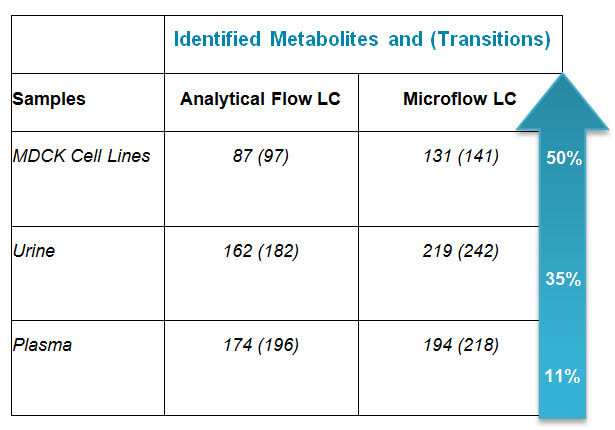
Why Microflow HILIC Chromatography for Targeted Metabolomics Applications?
I recently had the opportunity to catch up with Baljit Ubhi to discuss the top questions you’re asking in regards to using Microflow HILIIC Chromatography for Targeted Metabolomics. Here’s what Bal said:

Fipronil Tainted Eggs Detected in Several European Countries
News agencies all over the world are reporting a new food contamination issue regarding eggs which have been found to contain residues of Fipronil. According to Nieuwsuur, a Dutch news, and current affairs program, “The Fipronil scandal is a huge blow to the poultry sector. Millions of eggs are destroyed and 138 companies remain tentatively closed. But supermarkets also face great damage. In recent days all contaminated eggs have been taken out of the shelves.” CBS news has reported that contaminated eggs have been discovered in Belgium and in the Netherlands with other European countries now on alert.

In Search of the Unknown
The production of high-quality drinking water entails rigorous treatment and testing procedures. For water suppliers’ laboratories, such as the Zweckverband Landeswasserversorgung in Germany, one of the major challenges is the identification of trace levels of organic substances, which can be achieved with the help of mass spectrometry.

Discover The Benefits of Knowledge Base Articles
Did you know you can access Knowledge Base Articles for trending user questions compiled and answered by SCIEX support experts? Doing so may help to reduce your support calls, not to mention downtime. Instead of waiting for a problem to occur, you can stay on top of it, and be a part of the solution. To give you an idea of trending articles, consider the how this past month saw questions and answers including:

A Fleet of Analyzers Keeps Work Flowing
An Interview with Timothy Sangster, Head of Bioanalysis and Immunology, Charles River Laboratories, Edinburgh
Designed Specifically for Your Clinical Lab—Mass Spec Made Simple
Welcome to the first post in our clinical diagnostic blog series. Our ambition is to become your single destination for everything mass spec in the clinical diagnostic lab. To make this blog as useful as possible for you, we invite you to tell us what topics you would like us to cover. Please comment on this blog below and let us know what you’d like to hear!
Is Your Lab Prepared for Testing? The Global Supplement Market is Growing
Don’t judge a nutritional supplement by its label, as often, government monitoring of ingredients begins after the product enters the consumer market1. Meanwhile, there may be additional additives not mentioned on the label as they are used to address supplement side effects. Such is the case in the United States where even though federal law requires supplements to carry a dietary supplement label or a substitutional term, monitoring begins once a supplement is on the market. In China meanwhile, the China Food and Drug Administration’s (CFDA) health product potential illegal additives list, clearly stipulates monitoring processes for additives in six different types of nutritional supplements including weight loss, blood sugar reduction, blood pressure reduction, anti-fatigue, sleep improvement and immune strengthening functions.Read Tech Note >

How to Detect Additives in Cosmetics Amongst Ever Changing Regulations
In today’s technical blog, I’m talking about the cosmetics industry so let’s get right to it. According to a Research and Market report, “The Global Cosmetic market was $460 billion USD in 2014 and is estimated to reach 675 billion USD by 2020, growing at a rate of 6.4%.”1 The U.S. leads the pack with a reported $62 billion in revenue earned in 20162. So, what am I getting at? We know earnings are strong and consumers like their products. But the question remains, are these products that you put on your skin, hair, and ingest safe? Such is the thinking of scientists like me and other chemists who are routinely tasked with detecting minimal levels of potentially harmful ingredients in personal care products against ever-changing global regulations.

A Mine of Quantitative Proteomic Information
The Aebersold group at ETH Zurich focuses on proteomics research, including the development of techniques to study the proteome as an integrated entity. In collaboration with SCIEX, the group established SWATH® Acquisition mass spectrometry, a data-independent acquisition (DIA) method capable of fragmenting multiple peptide species concurrently. The resulting comprehensive data set can be retrospectively re-mined, enabling maximum benefit to be derived from any study.

Delivering New Biologics to the Marketplace
Characterization and quantification of host cell proteins (HCPs) in biopharmaceutical development and manufacturing is a critical step to ensuring product safety. While this can be achieved using ELISA, mass spectrometry using the SCIEX TripleTOF® 6600 System is more specific and enables the identification and quantitation of each of the individual proteins present.
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.

The Benefits of Using SWATH Acquisition Technology when Testing Pesticides in Food
Up until recently, SWATH® Independent Data Acquisition (IDA), was not widely used for the detection of pesticides in food samples. Introduced in 2012, SWATH Acquisition is an advanced acquisition technology capable of running on high-resolution mass spectrometers such as the X500R QTOF system or Triple TOF technology. Originally used in the Omics market to ID and quantify complex samples, SWATH Acquisition is gradually making a transition across markets including the investigation of pesticides in food. Like designer drugs, pesticides continuously undergo synthesizing, and food labs are beginning to require a more reliable analysis method to be confident in their resulting reports.

Single Injection, Routine Antibiotic Testing in Urine Samples
The consumption of pharmaceuticals and personal care products is a day to day occurrence. Once consumed the body excretes the remaining part of the compound which is not absorbed. This waste, flushed down the toilet, makes its way through the sewage system before arriving at a treatment facility where it was then processed with chemicals to ensure its cleanliness. Despite being washed, there can remain trace amounts of bacteria, hormones, metals, and antibiotics in whatever you consume, not just water
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.