This is the third and final post in our series in honor of International Women’s Day and our collaboration with World Cancer Research Fund. To wrap up, Deborah Howland talks about the importance of diet for anyone facing cancer – or trying to prevent it. A specialist...
Tags

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

Understanding technical debt: the hidden cost of ignoring problems
In today’s rapidly evolving technological landscape, businesses heavily rely on software and IT systems to drive their operations. However, the pursuit of efficiency and speed often leads to the accumulation of what is known as technical debt. Technical debt refers to the implied cost incurred when businesses choose quick but limited solutions over better approaches that may take more time to implement. This blog post will delve into the concept of technical debt, its implications for businesses and how to avoid falling victim to its detrimental effects.
Optimized rolling collision energy curves for IDA and SWATH DIA for peptides
During data dependent acquisition (DDA or IDA) or SWATH acquisition, the collision energy can be automatically adjusted according to the mass/charge and charge of the peptide. This dependency has been well characterized on our QTOF systems. By selecting rolling...

Rescheduling a Schedule I substance, and the Delta-8 controversy
Did you know that in the US, drugs and other chemicals are classified into 5 distinct categories depending on the drug’s acceptable medical use and its potential for abuse or dependency? Drugs federally classified as Schedule I substances by the US Drug Enforcement Administration (DEA) are considered to have the highest potential for abuse and for creating severe psychological and/or physical dependence. In addition to heroin, LSD and MDMA (ecstasy), cannabis is classified as a Schedule I substance in the Controlled Substance Act of 1970, which means it has no approved medical usage.
sMRM Pro Builder template tutorial
The sMRM Pro Builder template is an Excel-based tool that can help you implement large panels of analytes in your lab. The Excel sheet will take your preliminary experimental results and compute retention times, retention time window widths and dwell time weighting to optimize your targeted assay.

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.

Struggling to Analyze Small Volume Samples with Conventional LC-MS?
The M3 MicroLC System is designed for scientists who are struggling to analyze small volume samples with conventional LC-MS and need to lower their limits of quantitation while maintaining throughput and robustness. When designing the M3 MicroLC System, we...

Legal and Illicit Drugs in Wastewater Detected and Confirmed with QTRAP Technology
What happens when you up the sensitivity and lower detection limits on influent and effluent sewage tests? For starters, low levels of illegal drugs in samples begin to emerge. This is what researchers discovered when they combined the power of LC-MS/MS with the...
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...
Harnessing the Power of MRM3 for Large Molecule Quantitative Bioanalysis
In a previous blog outlining the advantages of high-resolution accurate mass measurements for protein quantitation using the TripleTOF 6600, it was noted that although the triple-stage quadrupole demonstrated high sensitivity when operated in multiple reaction monitoring mode (MRM), the relatively low-resolution measurement of m/z failed to discriminate Rituximab response from nominally isobaric interferences given the complexity of the proteolytically digested samples (June 28/2016). While the accurate mass filtering capabilities of the TripleTOF 6600 represents one mechanism for achieving increased selectivity over MRM, the triple quadrupole/linear ion trap (LIT) hybrid platform represented by the QTRAP® 4500, 5500, 6500 and 6500+ systems provides an alternative technique by leveraging a third stage of MS, often referred to as MRM3. In this blog, we outline the MRM3 scan function and survey several large molecule applications which utilize the additional stage of fragmentation in the LIT to yield significant improvements in achievable detection limits when compared to MRM.

A Sting in the Tale for Neonicotinoids
Did you know one out of every three mouthfuls of your meal is a product of honeybee pollination—almonds and other tree nuts, berries, fruits, vegetables? To put numbers behind it, honeybee pollination amounts to about $15 billion of U.S....
The detection of acid herbicides and urons by large volume injection
Pre-treatment versus direct injection – that is the question posed in the application note, “The Detection of Acidic Herbicides and Phenyl Ureas by LC-MS/MS with Large Volume Injection and Automated Column Switching.” It’s just one of the dozens of articles you will find within the Environmental Compendium (pages 1 to 4, pesticides) now available for download.
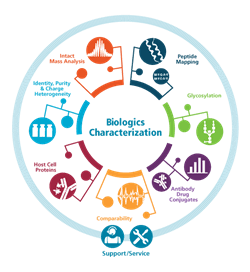
See What More You Can do With 360 Degree Biologics Characterization
Ever wish you had your own team of mass spectrometry experts at your side when working through biologics development and characterization challenges? With SCIEX 360° Innovations complex biologics characterization is streamlined with a full suite of mass spectrometry (MS), capillary electrophoresis (CE) systems, software, and services from SCIEX experts.

You’ve Seen It… Now Try It! BioPharmaView Software 2.0
At ASMS this year, the newest version of BioPharmaView Software was released. This software simplifies the processing of biotherapeutic data for characterization and comparability which can dramatically improve your productivity. BioPharmaView 2.0 Software accelerates characterization and comparability studies and simplifies reporting, so you can make better decisions, faster.

Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS
Quantitating antibiotics and insecticides in poultry is serious business. Overuse can lead to antibiotic resistance while insecticide residuals can cause harmful side effects in humans. In the United States, for example, the Federal Drug Administration (FDA), has offered up a plan to limit common antibiotics in feed, which are used to encourage growth. However, this is a voluntary plan, and as the following application note, “Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS,” points out, antibiotics have been shown to accumulate in poultry feathers, which are in turn used for nutritional elements in the feed.

Screening Novel Psychoactive Substances with Confidence
How do you know what you can’t see? This is the challenge many a lab faces as they relentlessly test for novel psychoactive substances (NPS) as unknown samples with an ever-changing ingredient list make discovery difficult work at best. There are many reasons for the complexities of which you can discover in this application note, “Accurate Mass Screening Workflows for the Analysis of Novel Psychoactive Substances.” However, the biggest of which is that non-targeted findings can turn up thousands of molecular features in a single sample. Sifting through the peaks is laborious, and many are normal besides.

Perfluoroalkyl Acids in Drinking Water – EPA Method 537
The United States Environmental Protection Agency (EPA), under the 1996 Safe Drinking Water Act (SDWA), requires a new list of no more than 30 unregulated contaminants to be monitored by public drinking water systems. Known as the Unregulated Contaminant Monitoring Rule (UCMR), a new list is published every five years. The last rule, UCMR3, was published May 2, 2012, and is the focus of the following application note, “Analysis of Perfluoroalkyl (PFFA) Acids Specified under the UCMR3 Using the QTRAP® 6500 LC-MS/MS system,” which can be found in the Food and Environmental Compendium.

SCIEX helps set food standards in China
One of the biggest concerns of Chinese citizens is food safety1. Even though China ranks second in global economies2, crowding, industrial pollution, labor and certain agriculture practices have contributed to this. In October 2015, however, we began to see a turnaround as the Chinese government revised its 2009 Food Safety Law in an attempt to strengthen its food supply oversight and quality.

Discover the new and accurate SCIEX way to enhance your routine food allergen testing
Food allergy is an immune-mediated, adverse reaction to an antigenic protein. Even limited exposure to an antigen can provoke a significant reaction in sensitive individuals, causing rashes, itching and swelling in the mouth, nausea, vomiting, and asthma. Additionally, food allergies are the leading cause of anaphylaxis, an acute, potentially deadly allergic reaction. The prevalence and severity of food allergies are rising, with approximately 150 million people suffering from food allergies worldwide.1, 2 Presently, there is no cure for food allergies, and sufferers must rely on the correct labeling of foods to avoid consuming allergens. Hence, the development of sensitive and accurate analytical methods to screen for the presence of allergens in food products is necessary for the prevention of potentially life-threatening health problems for allergy sufferers.

Three Infographics to Show You How to Overcome Challenges in Transitioning to Biologics Bioanalysis
The move to large molecules in Pharma is accelerating, offering unprecedented opportunities to improve human health and expand into new markets. But for those with extensive experience with small molecule bioanalysis, the shift to biologics can be challenging, from Sample Prep to Instrumentation and Software, to Methods and Training:

Rapid Characterization of Biologics using CESI-MS
Today, 30 monoclonal antibodies (mAbs), have been approved for the treatment of certain cancers, autoimmune and infectious diseases. Even more are in development, and perhaps you and your team of scientists are working on one now. Keeping pace with fast development timelines while performing comprehensive characterization of biologic candidates can be challenging. However, more and more, scientists are tackling these challenges with new techniques to speed and simplify their characterization workflows. Read more in the application note, “Rapid Characterization of Biologics using a CESI 8000 – SCIEX TripleTOF® System,” found in the Biologics Analytical Characterization Compendium, which highlights how CESI separation coupled with high-resolution mass spectrometry can provide a comprehensive characterization of biotherapeutics.

The Not So Hidden Truth about Climate Change How It’s Poisoning Your Food
Did you know climate change could be poisoning your food? According to the United Nations Environmental Program (UNEP) report on Emerging Issues of Environmental Concern, rising temperatures are making crops more toxic.

Guardians of Antibiotics
This second is a blog series on the global war: Rise of Superbugs! Part 1 took a critical look at the antibiotic threat we face in today’s battlefield. The waning effectiveness of antibiotics as we head into what may seem like a post-antibiotic era has impelled new reformation to at the very least control antibiotic usage to ensure food safety.
Protein Quantitation Workflows using the TripleTOF 6600: A Case Study for Rituximab
Although the triple-stage quadrupole (QQQ) mass spectrometer remains the pillar for quantitative LC-MS/MS bioanalytical assays, due in part to the platforms’ high duty cycle when operated in multiple-reaction monitoring (MRM) mode, the applicability of high-resolution mass spectrometry (HRMS) has become of increasing importance for protein quantitation given the complexity of proteolytically digested samples in the surrogate peptide approach. While the QQQ demonstrates high sensitivity and specificity, the relatively low-resolution measurement of m/z may fail to differentiate analyte response from nominally isobaric background interference. In contrast, HRMS with accurate mass assignment of product ion allows interference to be resolved through judicious selection of a post-acquisition mass extraction window whose tolerance is largely dictated by the effective resolution and stability of mass calibration.