We are thrilled to mark International Women’s Day 2019 by making our fourth annual donation to World Cancer Research Fund, a global not-for-profit organization and leading authority on the links between diet, nutrition, physical activity, and cancer. In fact, our...
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.

New features in OneOmics suite
I just wanted to thank the readers here, both the OneOmics suite users who’ve shared their time and watched OneOmics grow, and for all the talented developers and scientists who’ve made OneOmics suite what it is today.
Assess the performance of the Echo® MS system
To obtain the best, most reproducible results using the Echo MS system, it is important to select the best solvent for your analyte and matrix and to ensure the flow rate is optimized for your solvent. Please review this flow rate optimization community post to...
Merging two FASTA files
There is an easy way to merge two FASTA files together. Open notepad and type this string: type %*>"newfile.fasta" Enter any name for the FASTA file between the quotes. Save the file as: Merge FASTA files.bat Copy the two files you want to merge into the same...

Intabio acquisition expands SCIEX portfolio of impactful solutions to accelerate biotherapeutic development
Due to the nature of their production, biotherapeutics are difficult to manufacture. Growth conditions, purification protocols and formulation requirements can introduce unintended modifications into the protein structure that may affect its efficacy and safety.

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

Importing acquisition methods from Analyst software to SCIEX OS software
The SCIEX Triple Quad 7500 system is the first nominal mass instrument to be released completely on SCIEX OS software. Moving to a new software solution can be time consuming with the need to transfer numerous methods to the new platform. SCIEX OS software helps...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...

Discover the New X500B QTOF System, the Simpler, Faster Path to Biologics Characterization Answers
Have you ever wished for a compact instrument that delivers expert-level answers to your most complex biotherapeutic characterization challenges faster and easier than what you are doing now? At SCIEX, we recognize that even expert users want easier ways to perform daily characterization tasks and get great results every time. That’s why we set out to develop the X500B QTOF system: a robust and reliable new instrument and software solution that reduces complexity and simplifies biologics characterization workflows so every scientist can get expert-level results
How to Achieve Higher Sensitivity with Hybrid Immunoaffinity LC-MS Assays
Protein-based biotherapeutics, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs) are a growing component of pharmaceutical companies’ drug pipelines. The growth of ADCs in particular is due to their ability to selectivity target and deliver a potent molecule to a cancer cell based on a specific tumor marker. In order to support this growing class of new drug molecules, robust and reliable bioanalytical methods are required. While ligand binding assays (LBAs) like ELISA have been the most popular platform for biotherapeutic quantitation, bioanalytical scientists have been increasingly adopting hybrid LBA/LC-MS methods in this area.

The Application of Research Grade MetabolitePilot™ Software for the Determination of the Catabolic Peptide Products of Exenatide
The stability of peptide and protein biotherapeutics directly impacts their pharmacokinetic profile, efficacy, and safety. It is therefore essential to characterize the stability of a given bio-therapeutic including both in-vivo and in-vitro catabolism, thereby...

LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots
There is a lot you can tell from a droplet of blood as it’s snapshot of what could be present in a body at any given moment. In the following application note, LC-MS/MS Screening of 64 New Psychoactive Substances Using Dried Blood Spots, researchers did just...

LC-MS/MS Analysis of Emerging Food Contaminants
Ever wish you had access to the most up to date application methods but don’t know where to find them? The Food and Beverage Compendium is your one-stop resource for research notes ranging from pesticides, allergens, and antibiotics to mycotoxins, vitamins, and...

Is Your Beverage Truly 100% Fruit Juice?
There is nothing like the flavor of fruit juice whether freshly squeezed or made from concentrate to clench your thirst, except when it’s not 100 percent juice after all. As the following tech note, “Authenticity Assessment of Fruit Juices using LC-MS/MS and...
Superbugs, Antibiotic Resistance, and the QTRAP® 6500+ System
‘Superbugs’, or bacteria that have developed antibiotic resistance as a result of adapting to the drugs used in their treatment, are dangerous infections that doctors struggle to stop from spreading. Even common infections such as urinary tract infections and...
It’s Time to Enhance Your Food Testing
Are you looking for ways to up the ante on your LC-MS/MS when it comes to food testing? Researchers here have developed a method for the analysis of approximately 400 pesticides in food samples, and their work is available for viewing in this year’s compendium.

Top Five Misconceptions about Mass Spectrometry
Do you work in a lab handling precious samples yet, hesitant to make the move to mass spectrometry? Many laboratories just like yours continue to conduct sample analysis using ELISA assays, PCR scans, and amino acid tests because of their effectiveness. These processes work, so why change? Well, these type of analytical experiments can report false positive and negative results. You have trained your staff, know the process, and fingers crossed, not too many user errors have compromised analysis.

Rapid Separation Method for Intact Monoclonal Antibodies (Mab) Merges Charge Variant, Impurity, and Glycoform Analyses into a Single Assay
Throughout all stages of development and manufacture, monoclonal antibodies (mAbs) exhibit a great deal of structural complexity. After translation and folding, proteins undergo post-translational modifications, as well as spontaneous and enzymatic degradation, such that a single preparation of purified mAb exhibits a range of small structural changes, composed of various glycoforms and charge variants, as well as amino acids alterations due to oxidation, deamidation, isomerization, or other chemical reactions. This display of structural heterogeneity can influence the overall stability, efficacy, and safety profile; therefore, understanding the extent of structural modifications has become extremely important to drug manufacturers who continually assess mAb composition throughout bioprocessing to demonstrate stability, batch-to-batch consistency, and long-term shelf life.

Glycosylation Analysis Designed for the (Protein) Masses
A variety of post-translational modifications (PTMs) can impact a biotherapeutic protein’s mass, but none are as common as glycosylation.[1] Hence, the headline for a recent article in Genetic Engineering and Biotechnology News, “Post-Translational Icing on the Biologics Cake,” featuring comments from Sean McCarthy, Ph.D., Global Market Manager of Biologics at SCIEX.

Struggling to Analyze Small Volume Samples with Conventional LC-MS?
The M3 MicroLC System is designed for scientists who are struggling to analyze small volume samples with conventional LC-MS and need to lower their limits of quantitation while maintaining throughput and robustness. When designing the M3 MicroLC System, we...

Legal and Illicit Drugs in Wastewater Detected and Confirmed with QTRAP Technology
What happens when you up the sensitivity and lower detection limits on influent and effluent sewage tests? For starters, low levels of illegal drugs in samples begin to emerge. This is what researchers discovered when they combined the power of LC-MS/MS with the...
Using SelexION to Increase Selectivity for the Accurate and Sensitive Quantitation of a Difficult Peptide Therapeutic
SelexION® DMS Technology Drives Advancements in Challenging Large Molecule Bioanalysis By the End of 2024, the peptide therapeutics market value is expected to reach US$46.6 billion1. However, peptide therapeutics present some of the toughest analytical...
Harnessing the Power of MRM3 for Large Molecule Quantitative Bioanalysis
In a previous blog outlining the advantages of high-resolution accurate mass measurements for protein quantitation using the TripleTOF 6600, it was noted that although the triple-stage quadrupole demonstrated high sensitivity when operated in multiple reaction monitoring mode (MRM), the relatively low-resolution measurement of m/z failed to discriminate Rituximab response from nominally isobaric interferences given the complexity of the proteolytically digested samples (June 28/2016). While the accurate mass filtering capabilities of the TripleTOF 6600 represents one mechanism for achieving increased selectivity over MRM, the triple quadrupole/linear ion trap (LIT) hybrid platform represented by the QTRAP® 4500, 5500, 6500 and 6500+ systems provides an alternative technique by leveraging a third stage of MS, often referred to as MRM3. In this blog, we outline the MRM3 scan function and survey several large molecule applications which utilize the additional stage of fragmentation in the LIT to yield significant improvements in achievable detection limits when compared to MRM.

A Sting in the Tale for Neonicotinoids
Did you know one out of every three mouthfuls of your meal is a product of honeybee pollination—almonds and other tree nuts, berries, fruits, vegetables? To put numbers behind it, honeybee pollination amounts to about $15 billion of U.S....
The detection of acid herbicides and urons by large volume injection
Pre-treatment versus direct injection – that is the question posed in the application note, “The Detection of Acidic Herbicides and Phenyl Ureas by LC-MS/MS with Large Volume Injection and Automated Column Switching.” It’s just one of the dozens of articles you will find within the Environmental Compendium (pages 1 to 4, pesticides) now available for download.
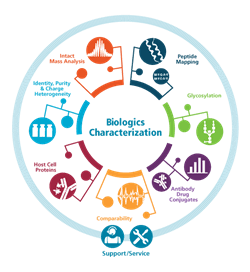
See What More You Can do With 360 Degree Biologics Characterization
Ever wish you had your own team of mass spectrometry experts at your side when working through biologics development and characterization challenges? With SCIEX 360° Innovations complex biologics characterization is streamlined with a full suite of mass spectrometry (MS), capillary electrophoresis (CE) systems, software, and services from SCIEX experts.

You’ve Seen It… Now Try It! BioPharmaView Software 2.0
At ASMS this year, the newest version of BioPharmaView Software was released. This software simplifies the processing of biotherapeutic data for characterization and comparability which can dramatically improve your productivity. BioPharmaView 2.0 Software accelerates characterization and comparability studies and simplifies reporting, so you can make better decisions, faster.

Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS
Quantitating antibiotics and insecticides in poultry is serious business. Overuse can lead to antibiotic resistance while insecticide residuals can cause harmful side effects in humans. In the United States, for example, the Federal Drug Administration (FDA), has offered up a plan to limit common antibiotics in feed, which are used to encourage growth. However, this is a voluntary plan, and as the following application note, “Quantitation of Antibiotics and Insecticides in Poultry Feed using LC-MS/MS,” points out, antibiotics have been shown to accumulate in poultry feathers, which are in turn used for nutritional elements in the feed.