Elevate Performance in Your Forensic Toxicology LabYou only need to skim through the United Nations World Drug Report 2018 to see that drug abuse is escalating. Associated with numerous medical, social, and legal problems, it comes as no surprise that ‘drugs of abuse’...
Tags

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
Questions and answers to help improve your mycotoxin analysis
During a recent webinar I shared method details for mycotoxin analysis on the SCIEX 7500 system. In this blog i will share the Q&A for the submitted questions that we did not have chance to answer during the live webinar.

Guide decisions during cell line development with more information at the intact level
Monitoring product quality attributes (PQAs) throughout monoclonal antibody (mAb) development is vital to ensuring drug safety and efficacy. By adopting orthogonal analytical techniques and integrating new technologies that have the potential to provide more information, it is possible to improve product quality and manufacturing efficiency and make more informed decisions.

Unlock the benefits of nominal mass spectrometry for NPS analysis
The development of analytical methods for the detection and quantitation of drugs and metabolites in a range of biological matrices is a challenging process. Forensic toxicology labs need a reproducible and reliable methodology to ensure the robustness of the data and the quality of the results. They also need robust and sensitive instrumentation that can detect drugs at trace levels with high specificity, especially when it comes to novel psychoactive substances (NPS), which can be difficult to monitor and control.

Metabolite identification and peace of mind
Managing metabolite identification (Met ID) studies is challenging, so what is at the top of your priority list as you plan the year ahead? Ensuring you have the data needed to manage product safety, meeting deadlines, staff recruitment and training, maintaining compliance, capital expenses, or something else?

Acoustic Ejection Mass Spectrometry at ASMS 2023
For all those interested in learning about all the cool applications possible with AEMS and the Echo MS system, here is a list of presentations at ASMS 2023. Monday: MOH 3:50pm – Intact protein analysis MP 294 – Metabolite identification MP 312 Compound QC Tuesday TOB...
Speeding the Development of Quantitative Biosimilar Assays
When developing new quantitative assays for Biotherapeutics, every biologic requires a specific sample prep strategy, which includes sourcing reagents and research protocols. However, as every bioanalytical lab knows all too well, it can also take up to two months to develop an optimized and robust LC-MS assay. For this reason, researchers understandably want an easier way to develop highly sensitive and specific assays for biotherapeutics and biosimilars to accelerate sample turnaround time.

The Benefits of Using SWATH Acquisition Technology when Testing Pesticides in Food
Up until recently, SWATH® Independent Data Acquisition (IDA), was not widely used for the detection of pesticides in food samples. Introduced in 2012, SWATH Acquisition is an advanced acquisition technology capable of running on high-resolution mass spectrometers such as the X500R QTOF system or Triple TOF technology. Originally used in the Omics market to ID and quantify complex samples, SWATH Acquisition is gradually making a transition across markets including the investigation of pesticides in food. Like designer drugs, pesticides continuously undergo synthesizing, and food labs are beginning to require a more reliable analysis method to be confident in their resulting reports.

Single Injection, Routine Antibiotic Testing in Urine Samples
The consumption of pharmaceuticals and personal care products is a day to day occurrence. Once consumed the body excretes the remaining part of the compound which is not absorbed. This waste, flushed down the toilet, makes its way through the sewage system before arriving at a treatment facility where it was then processed with chemicals to ensure its cleanliness. Despite being washed, there can remain trace amounts of bacteria, hormones, metals, and antibiotics in whatever you consume, not just water
Do You Want to Accelerate Quantitative Assays for Antibody Drug Conjugates?
Are you tasked with the bioanalysis of antibody drug conjugates (ADCs)? If so, you know they represent a rapidly growing class of biotherapeutics, but their unique chemical structure makes quantitative analysis particularly challenging.

Food Allergies – How Allergic Are You?
A recent study published by the Annals of Allergy, Asthma, and Immunology (ACAAI), pointed out, in a study of 109 people tested, that skin prick tests are not 100 percent reliable. In the study, participants were subjected to oral food challenges prior to skin testing in which 50 percent of individuals had no reaction. It was also discovered that blood tests were not full-proof even though they measure the presence of IgE antibodies to specific foods. These results are not surprising given that 50 to 60 percent of tests result in false-positives.
Comprehensive Therapeutic Protein Characterization Using One Single Method
Get to know how CESI-MS will allow you to quickly and accurately characterize protein therapeutics for attributes in a single method by downloading this discovery kit.

A Fresh Approach to Food Safety
The EU Reference Laboratory (EURL) for Fruits and Vegetables in Almería is responsible for a network of around 200 laboratories which provide essential surveillance and monitoring to ensure the safety of foodstuffs available across Europe. The EURL provides proficiency testing and method development for these official laboratories, ensuring rigorous screening to avoid harmful chemicals entering the food chain
The Only Solution You Need for Fast MetID
If you work in the breakneck world of therapeutic development, then you probably don’t even have time to read this blog (but we thought we would write it anyways, just in case). Drug metabolism samples are coming into your lab fast and furious. You need to turn them around in hours so that chemists and biologists can optimize the effectiveness of the therapeutic candidate. Time for lunch? We don’t think so!

How Easy Is It to Relocate a Mass Spectrometer?
When you’re in the process of moving your lab, across the corridor or to another country, there’s a lot to think about. Adding to the stress, there’s not always a lot of time to plan, or budget allocated for the process, especially in the case of unexpected urgent maintenance work.

The Key to Measuring Chemical Dyes in Food is LC-MS/MS
Adding colorful dyes to food is nothing new. In the early 19th century, for example, it wasn’t uncommon for manufacturers to add chalk to white bread, thicken milk with a lead compound, and inject red dye into meat in the quest for a fresher appearance1. Fast forward to the 21st century, however, and along with mass spectrometry, food standards have come a long way. Foods now must pass muster according to standards set by government regulators or else risk fines and punishment which can be costly for the manufacturer. To support these measures, are agencies such as the US-FDA, EFSA, and others which have banned some colors due to their toxic and carcinogenic nature which brings me to mass spectrometry analysis. Discover more when you read the following application note, “LC-MS/MS Analysis of Emerging Food Contaminants,” in which researchers used the ExionLC AD with a Phenomenex Column for sample separation followed by MS/MS detection with the SCIEX X500R QTOF system.

What is your Method for Separating Challenging Polar Molecules?
From small ions like phosphate, herbicide degradation to metabolites, oligosaccharides, peptides, and proteins. How is your lab analyzing polar molecules? The reason I ask is there is a saying, if you have a charged or polar molecule, look to capillary electrophoresis (CE) first. While liquid chromatography (LC) is an ideal front-end separation tool for many types of molecules, as the following poster points out, “From Small to Very Large, Orthogonal, Sensitive Polar Molecule Analysis by CESI-MS,” there are some situations that call for CE over LC analysis. For those of you that are not familiar with CESI-MS, it is the combining of CE separation with electrospray ionization, into one dynamic process, within the same device.

The Trouble with PFAS in Drinking Water
There has been a string of news articles concerning polyfluorinated alkyl substances (PFAS) in drinking water these days, and I must say, they have my attention. Here is the thing, when you think of drinking water in the United States, for example, crystal clear lakes, rivers, and groundwater along with effective water treatment come to mind. On the flip side, as safe, some supplies may be there are communities such as that of Flint, Michigan, which have been dealing with lead filled pipes for far too long. Contamination was so bad there that residents were provided bottled water for drinking purposes as the state decided who was responsible for replacing the affected water lines.

SWATH Acquisition – Master of All Trades
SWATH® Acquisition is an innovative strategy for acquiring data on a TripleTOF® mass spectrometer. In a previous blog, we learned how SWATH works. Now let’s learn what it can do for different applications:
The Power Behind LC-MS for Quantifying mAb Therapeutics
Quantitation of monoclonal antibodies (mAbs) in biological fluids is important during all stages of antibody drug development. First developed in the 1970s, therapeutic mAbs have both research and medicinal impact as they can be used for diagnosis and treatment of a wide variety of diseases, and have a high level of specificity.

Data Independent Acquisition Mass Spectrometry with the Power of SWATH
There are many different methods in use today to acquire data on a mass spectrometer, but few have generated as much buzz in recent years as SWATH technology. First reported 5 years ago by Ruedi Aebersold and his group1, SWATH® Acquisition on a TripleTOF® instrument has rapidly become one of the premier acquisition strategies for identification and quantitation of complex samples. But what exactly is SWATH and why is it so powerful? In order to answer these questions, let’s first take a step back and look at the larger picture.
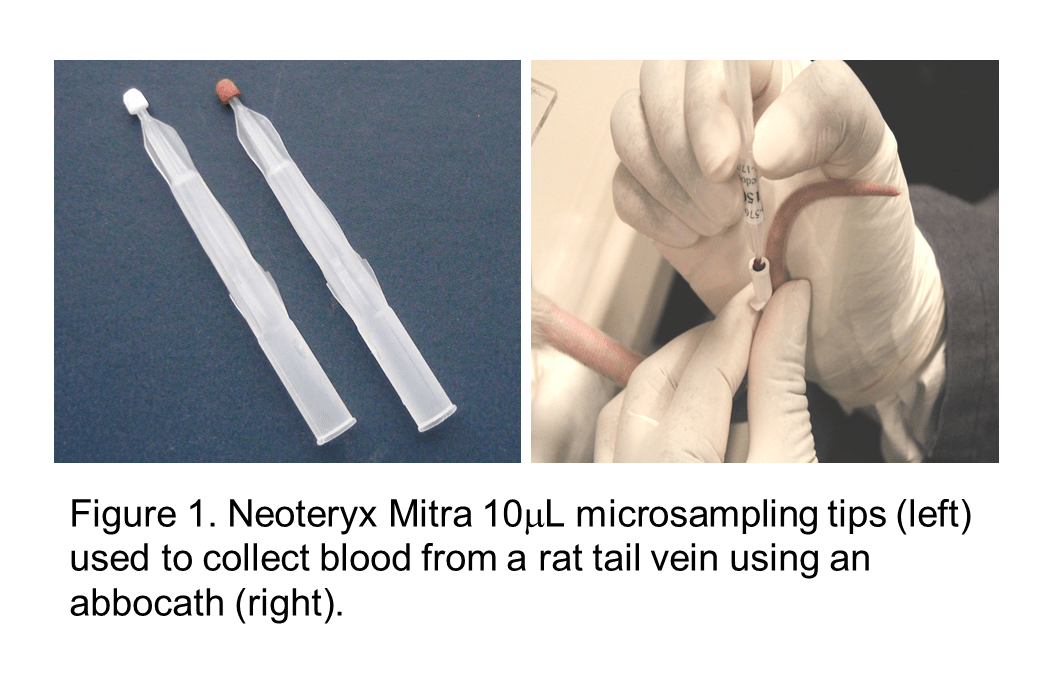
Volumetric Absorptive Microsampling and the SCIEX 6500+: A Pre-Clinical Case Study for the Biotherapeutic Exenatide
In an effort to Replace, Refine, and Reduce the number of animals used for pre-clinical research, several microsampling strategies have been implemented which allow for the consolidation of satellite TK and main study groups. In addition to the ethical gains driven by these 3Rs, microsampling has the potential of increasing scientific value since it becomes feasible to directly correlate exposure, toxicological effects and pharmacological response in the same individual
Say Goodbye to MetID Headaches, Say Hello to Automated Large Molecule Catabolism Processing
Is your mind swimming with large molecule catabolism data? Do you spend hours manually processing through complex spreadsheets to match your data with theoretical biologic catabolites? Are you wasting precious time by drawing out and processing your therapeutic peptide as a small molecule? Are you worried that you could be missing something?

Using the X500R QTOF System and SCIEX OS to Identify and Quantify Food Residues
Farmers use pesticides to protect crops from insects and disease as pesticides are necessary to create the volume of food that our population requires. Without them, we would not be able to grow enough crops to feed the world—they are a necessary evil. Government agencies such the Food and Drug Administration (FDA) in the United States and pesticide manufacturers, however, work hard to educate farmers on how to minimize their use. However, sometimes farmers add too much which leaves a residue. Upon harvest, farmers wash the fruit and vegetables. Once complete the crop then makes its way to the wholesaler and eventually, the supermarket. Even so, there may still be pesticide residue on the produce, which is why government agencies randomly pull produce from store shelves for testing of maximum residual limits (MRLs) and we are encouraged to wash our food before consuming it

The World Has its Eyes on Precision Medicine
What if we could understand and then treat diseases on an individualized level, in a way that was tuned to a person’s individual biology? Not in a futuristic, ‘wave a high-tech scanner across a person’s body’ way, but in a legitimate ’I can run a lab test and know the best action to take’ way. This is the promise of Precision Medicine, to deliver the right treatment to the right patient, at the right time, predicting more accurately which treatments will work for certain groups of patients, in contrast to the pervasive one-size-fits-all approach. More specifically, if we could provide a comprehensive report at the molecular level of an individual (based on genome, proteome, or metabolome profiles), a physician could be much better informed to make optimal treatment decisions. And if we could track these profiles over time, a person could adjust their lifestyle to focus on long-term wellness.

Setting Records with Fast Glycan Technology
There is a lot of talk going around in the lab, and it has to do with the newly released Fast Glycan Labeling and Analysis technology. Where once research analysts needed to set aside days to perform glycan analysis, now takes an hour or so. Glycans are immediately identified by the software – so no need for data interpretation. That’s up to 5x faster than HILIC.