Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
Tags
Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.
6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?

An overview: LC-MS analysis of targeted protein degraders and their metabolites
Targeted protein degraders (TPD) are a relatively new therapeutic modality that opens the potential to target disease-causing proteins. These disease-causing proteins have been highly challenging for traditional small-molecule therapeutics to treat, making TPDs an exciting new therapeutic modality.

Advanced analytics for mAb variants
Access mab-variants-infokit>
High level method optimization considerations for Echo MS system
While an in-depth discussion of method development and optimization for the Echo® MS system is beyond the scope of a community post, here are some points to consider as part of the process: The maximum recommended ion spray voltage for prolonged electrode life is 5000...
Tips to maximize electrode lifetime for Echo MS system
While it’s easy to think of the Echo® MS system as an ultrafast LC system in front of the SCIEX Triple Quad 6500+ mass spectrometer, the system operates on fundamentally different principles. For this reason, it requires different routine maintenance to keep it...
Uploading and using transcriptomics data in the OneOmics suite
RNA experiments can be created in the OneOmics suite for multi-omics analyses, enabling integration of transcriptomics data and proteomics data for biological insight. To build RNA experiments, either CloudConnect for PeakView software 2.2 or BaseSpace (Illumina) can...
High complexity of the lipidome
The complexity of the lipidome is diverse in the structure and there are many combinatorial isoforms that are available within nature. Currently, many different techniques are required to fully characterize a lipid molecule. What if you could do it in a single...
What is the difference between MRM3 vs MS/MS/MS (MS3)?
The MRM3 workflow and the MS3 scan are functionally the same QTRAP system scan, but used with different goals in mind. The main difference is how these scans are used in the whole MS workflow. With MS3 scans, you can use these in a data dependent mode for discovery...
Standard addition workflow – for quantification and calculating background levels
The method of standard addition is a quantitative analysis approach used in situations where matrix effects from complex samples contributes to the analytical signal. This makes it impossible to compare the analytical signal between sample and standard using a...
How do I define the experimental design (the metadata) for my SWATH acquisition study within the OneOmics suite? What are the requirement for replicates?
In quantitative Omics research, the goal is to understand which analytes (protein or metabolite) are perturbed between experimental conditions; therefore we carefully design our studies to explore these questions. The algorithms used within the Assembler application...
Controlling the M5 MicroLC system with SCIEX OS software using contact closure
Contact closure can be used to control external devices that are not directly controlled by SCIEX OS software. A sample batch is first created in the SCIEX OS software for MS acquisition, and then a similar batch is created on an external LC device with the required...
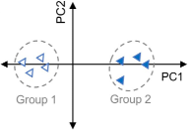
What are my normalization options in MarkerView software and when should I use them?
In an LC-MS experiment there are multiple sources of variance that can confound the quality of your results. This variation can be biological e.g. differences between treated and control groups, but can also be non-biological, usually from small variations in...

Intabio acquisition expands SCIEX portfolio of impactful solutions to accelerate biotherapeutic development
Due to the nature of their production, biotherapeutics are difficult to manufacture. Growth conditions, purification protocols and formulation requirements can introduce unintended modifications into the protein structure that may affect its efficacy and safety.

Top questions about the exposome of PFAS revealed
According to the CDC, the exposome is “the measure of all the exposures of an individual in a lifetime and how those exposures relate to health.”

What’s in your citrus oil?
Craig Butt explains a non-targeted omics approach to characterizing and profiling compounds in citrus oil Read time: 4 minutes There is increasing interest among consumers in the benefits of natural products containing citrus beyond the traditionally known benefits of...

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

Importing acquisition methods from Analyst software to SCIEX OS software
The SCIEX Triple Quad 7500 system is the first nominal mass instrument to be released completely on SCIEX OS software. Moving to a new software solution can be time consuming with the need to transfer numerous methods to the new platform. SCIEX OS software helps...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...

The top 5 questions to ask when investing in accurate mass technology for forensic toxicology workflows
Are you considering the purchase of a high-resolution accurate mass (HRAM) instrument for your forensic toxicology lab? To help ensure you invest in a solution that ideally meets your needs, ask yourself the following key questions. 1. How do I ensure my results...

Innovation that’s blasting through limitations in explosive detection
Mass spectrometry’s important role in identifying explosives The need for rapid explosive detection is now an unfortunate reality. The remit is multifaceted. The first is for preventative purposes, to protect us from any threat to life. The second is in the...
A new generation of therapeutic modalities
There are over 7,000 genetic diseases that could potentially be cured using gene therapy. Rare metabolic diseases, autoimmune disorders, cardiovascular disease and cancers are some of the top disease classes that can be addressed with gene therapies. With over 1,000...
Enhancing Biologics with CESI-MS Characterization
Comprehensive characterization of a biologic requires analysis at both the intact and digest levels, but these analyses can be complex and cumbersome. For example, with conventional liquid chromatography separations, researchers are often left with limited information...