Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.
Tags

Pharma perspectives: The influence of LC-MS innovation on drug development outsourcing
It is no secret that (bio)pharmaceutical research and development is complex, both scientific and regulatory processes. Working for a contract research organization and more recently for SCIEX has provided an interesting perspective on trends the market experiences that affect many of us.
Overcoming uncertainty in your PFAS analysis
Just like gum on the bottom of a shoe, the existence of per- and poly-fluorinated alkyl substances (PFAS) in our environment is a sticky one. If you’re in the field of environmental testing, then you’re all too familiar with the threat these substances have on public health. While we have learned a lot about them over the years, there is still much more to understand. With the right detection methods, we can gather the information we need to empower us to make informed decisions on reducing the risks they impose.

6 Signs it’s time for a new vendor
A lab’s success depends on many factors from instrument quality to efficient operations, including being partnered with the right vendor. A vendor is more than just a supplier. They should provide you with a high-level quality of support in maximizing the lifespan and performance of your systems, reducing downtime, enhancing ROI and more. How do you know if you’re partnered with the right one? Here are six signs it might be time to find someone new.

Selecting an LC-MS system for quantitation of pharmaceutical drug development
We understand you are busy, needing to prioritize running instruments, reporting results and managing your laboratory to meet deadlines. We created a solution guide to explain how SCIEX systems fit in the drug development pipeline to save you time evaluating options.

Nitrosamines: Where are we now?
Nitrosamines are a large group of N-nitroso compounds that share a common functional N-N=O group. They are produced by a chemical reaction between a nitrosating agent and a secondary or tertiary amine. Back in 2018, nitrosamines suddenly found themselves in the spotlight when they were unexpectedly detected in medications for high blood pressure. Since then, they have been found in several other prescription medications, including those for heartburn, acid reflux and diabetes, resulting in manufacturers recalling some common medications.

Celebrating customer experience: Insights from SCIEX leaders
Introduction Customer Experience Day (CX Day) is a special occasion for SCIEX, celebrated every first Tuesday in October. It’s a day dedicated to recognizing the incredible value of our customers and the relentless dedication of our associates who strive to make...

PFAS analysis in food: a robustness study in sensitivity and stability
The combination of per- and polyfluoroalkyl substances (PFAS) testing, trace-level regulatory requirements and complex MS applications can be intimidating. In a recent webinar, now available on demand, SCIEX PFAS expert Craig Butt demonstrated how the new SCIEX 7500+ system can help make PFAS testing easier.

Your success and voice go a long way!
At the heart of everything we do is ensuring that your workflows and team are empowered to achieve optimal results with your SCIEX instruments, software, consumables, and services. Every interaction with SCIEX is designed to support your success through the dedication...

FDA’s final rule on LDTs: what does it mean for clinical laboratories?
On April 29, 2024, the U.S. Food and Drug Administration (FDA) announced a final rule regulating laboratory developed tests (LDTs) as in vitro diagnostic devices (IVDs) under the Federal Food, Drug and Cosmetic Act (FD&C Act). This rule amends FDA’s regulations to state that in vitro diagnostic tests “manufactured” by clinical laboratories fall within the scope of the FDA regulatory oversight and is poised to dramatically shift the way clinical diagnostic laboratories in the United States develop and offer LDTs in the future. Read this blog post for a basic overview of the scope, intent and implications of this final rule, including the regulatory requirements, exceptions and timeline for implementation.

LC-MS system replacement: Are you ready?
Meeting deadlines in a bioanalysis laboratory can be a big challenge. Older, less sensitive and less reliable LC-MS systems make it even more difficult. Even the disruption caused by the installation and validation can be disconcerting and delay decisions. Does this sound familiar?
Assess the performance of the Echo® MS system
To obtain the best, most reproducible results using the Echo MS system, it is important to select the best solvent for your analyte and matrix and to ensure the flow rate is optimized for your solvent. Please review this flow rate optimization community post to...

Back to the new basics: Part 1 | Making the leap from GC-MS to LC-MS
Producing accurate results quickly in a demanding environment is no easy feat for analytical scientists. What’s more, many of us are constantly questioning ourselves—I certainly am—about whether we are employing the best technique for the analysis at hand.
It’s an overwhelming thought, considering the wide range of tools that are available to choose from, each of which offers varying levels of capacity, sensitivity, selectivity, specificity and cost. How do you meet the unique needs of your organization without breaking the bank? I get it, and I’m not here to convince you it’s easy. My aim is to guide you through the process to help you make the right decision for you.
How to optimize sample plating to run Echo MS system in ‘Fast acquisition mode’
The Echo MS system can acquire data extremely rapidly, at 1 second per sample. To achieve this speed, it should be ensured that there is adequate time between ejections for the analyte signal to return to baseline between ejections. Plating your samples such that you...
Uploading data for use in OneOmics suite
There are two options for data storage when working with the OneOmics suite. You can store your data either within the SCIEX Cloud platform in the Data Store, or you can store data in BaseSpace (Illumina) and link your BaseSpace account with the SCIEX Cloud platform....
MRM method transfer from a SCIEX Triple Quad or QTRAP 6500+ system to the SCIEX 7500 system
General recommendations when beginning method development Objective: The purpose of this document is to provide a quick reference for transferring MRM-based quantification methods from a SCIEX Triple Quad or QTRAP 6500+ system to a SCIEX 7500 system. While the best...
Save time by saving your workspace layout in SCIEX OS software
This video teaches you how to save your workspace layout in Analytics section of SCIEX OS software. Streamline your data visualization by saving optimized workspace layouts for your various data processing workflows. RUO-MKT-18-13321-A
Adapting a SCIEX high flow source for microflow LC
To set up a SCIEX high flow source for microflow LC (Turbo V ion source, DuoSpray source or IonDrive Turbo V ion source), first you must replace the wider bore electrodes with more narrow bore hybrid electrodes. Note with the OptiFlow Turbo V ion sources, there are...

Thailand cannabis legalization
Thailand has become the first southeast Asian country to legalize cannabis for medical use. Cannabis was originally introduced into Thailand from India, and until it was outlawed in the 1930s, it was historically used as a kitchen condiment, medicine and source of fiber.
High level method optimization considerations for Echo MS system
While an in-depth discussion of method development and optimization for the Echo® MS system is beyond the scope of a community post, here are some points to consider as part of the process: The maximum recommended ion spray voltage for prolonged electrode life is 5000...
Uploading and using transcriptomics data in the OneOmics suite
RNA experiments can be created in the OneOmics suite for multi-omics analyses, enabling integration of transcriptomics data and proteomics data for biological insight. To build RNA experiments, either CloudConnect for PeakView software 2.2 or BaseSpace (Illumina) can...
Stay informed on the latest novel psychoactive substances (NPS) flooding the US drug market
Each year, the Drug Enforcement Administration (DEA) Special Testing and Research Laboratory publishes an Emerging Threat Report. The data contained in the report is updated quarterly and represents a snapshot of the drug evidence seized and analyzed by the DEA in the...
Standard addition workflow – for quantification and calculating background levels
The method of standard addition is a quantitative analysis approach used in situations where matrix effects from complex samples contributes to the analytical signal. This makes it impossible to compare the analytical signal between sample and standard using a...
How do I define the experimental design (the metadata) for my SWATH acquisition study within the OneOmics suite? What are the requirement for replicates?
In quantitative Omics research, the goal is to understand which analytes (protein or metabolite) are perturbed between experimental conditions; therefore we carefully design our studies to explore these questions. The algorithms used within the Assembler application...
Downloading results from SCIEX Data Store or BaseSpace
Once your data processing sessions have completed, the results files are saved back to either SCIEX Data Store or BaseSpace. These can be downloaded from the cloud to your desktop for additional analysis. Please see these community posts to learn more: Explaining the...
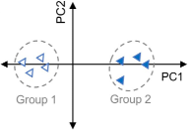
What are my normalization options in MarkerView software and when should I use them?
In an LC-MS experiment there are multiple sources of variance that can confound the quality of your results. This variation can be biological e.g. differences between treated and control groups, but can also be non-biological, usually from small variations in...
Quickly compare identification results from ProteinPilot software
When a ProteinPilot Software search is complete, a ProteinPilot report is generated that contains all the false discovery rate (FDR) analysis information. More information on using the large and small ProteinPilot reports can be found here When doing method...
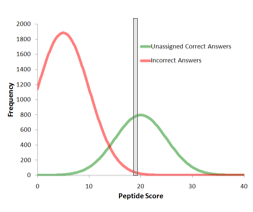
Computing protein confidence with improved accuracy by reassessing peptide confidence during protein grouping
ProteinPilot software 4.0 and higher releases include a new method for calculating protein confidences that improves reliability at the end of protein lists. Figure 1 shows a simulated example that demonstrates how more accurate protein confidence is computed. Figure...

Top questions about the exposome of PFAS revealed
According to the CDC, the exposome is “the measure of all the exposures of an individual in a lifetime and how those exposures relate to health.”

Breaking down the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Sensitivity and robustness carry different meanings in the world of mass spectrometry. Generally, sensitivity refers to an instrument’s ability to achieve lower limits of detection (LOD). Robustness, on the other hand, refers to an instrument’s ability to consistently...

The honey sting
As a consumer it’s hard for me not to feel inundated with claims that our food is “all-natural” or “chemical-free” or that we should buy certain “superfoods” for their health benefits. We read labels and trust that the product we are buying is what we are truly...